A light-activated metal–organic capsule enables the selective purification of progesterone from similar steroidal structures, even at five-fold excesses. The researchers who developed the capsule say that it could be modified for use with other compound classes and ultimately provide a greener alternative to conventional separation techniques.
Over one million tonnes of progesterone and related hormonal steroids are manufactured globally each year. These structurally complex drugs, which play an important role in female reproductive health, are typically produced by semisynthesis, but separation of the product compounds from similar precursors and side products is challenging. ‘It’s a very well-optimised process, but it’s still a very expensive process involving the use of very large volumes of chromatographic supports and solvent,’ says Jonathan Nitschke, a supramolecular chemist at the University of Cambridge, UK. ‘Chemical purification takes 10 to 15% of the global energy budget, so anything we can do to improve that will have a real impact.’
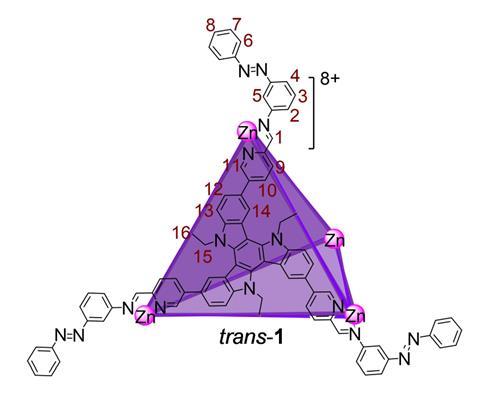
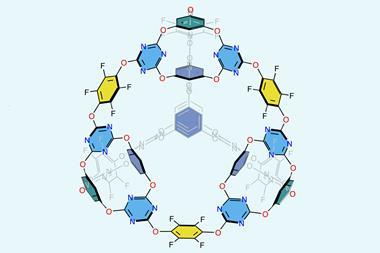
Metal–organic cages are a potential low-energy and low-solvent alternative to conventional purification techniques, instead relying on the highly selective binding of target molecules in a self-assembled cavity to separate mixtures. Now, Nitschke and postdoc Amit Ghosh have combined this molecular recognition approach with a light-controlled metal–organic cage, allowing them to selectively separate progesterone from similar steroid structures in a reversible and repeatable purification cycle.
The cage contains twelve trans-diazo groups, coordinated around four zinc atoms to form a tetrahedron with a cavity perfectly tailored to accommodate progesterone. When irradiated at 350nm, the diazo groups isomerise into the bulkier cis configuration, forcing some of the coordinated nitrogen atoms to dissociate from the zinc and causing the cage to open. Irradiation at 500nm then restores the trans configuration, re-forming the cage.
Exciting possibilities
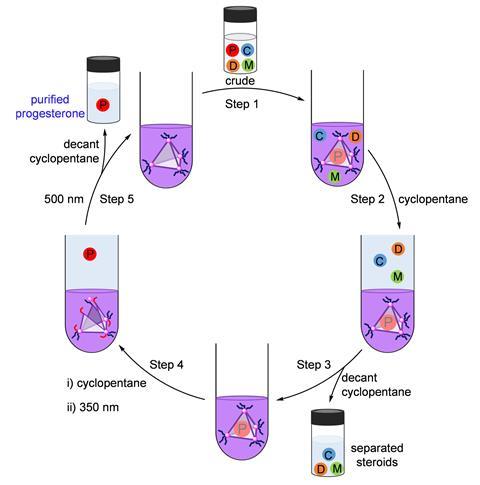
The team used a biphasic mixture of cyclopentane and acetonitrile to extract the different components at each stage of this cycle. ‘In acetonitrile the progesterone binds within the cage cavity but the other steroids are outside in the solution. When we add cyclopentane, the free steroids move into that layer which we can decant,’ explains Ghosh. ‘Irradiation then releases the progesterone from the cavity and we can dissolve it in cyclopentane to separate it from the cage.’ The team recovered 73% of the progesterone in the mixture, successfully separating the compound from the similar steroids mestranol, cholesterol, and 7-dehydrocholesterol in up to five-fold excesses.
Although still at the proof-of-concept stage, other researchers in the field believe that the work already offers exciting possibilities. ‘Conceptually it’s really interesting. I think it’s more suited towards the fine chemical end of the spectrum, rather than bulk purification but these cages could also have applications in analytical chemistry and detection,’ says Ben Pilgrim, a supramolecular chemist at the University of Nottingham, UK. ‘It would be interesting to see how generally applicable this concept of using a photoswitch to dissociate cage corners is to other cages, other applications, and even in water.’
The Cambridge team are keen to explore the full potential of this method and ultimately hope to develop further cages for other important compounds. ‘We’d need to scale it up quite considerably for any industrial applications and we’d like to talk to process chemists about the practicalities and costing the process,’ says Nitschke. ‘We’re also thinking about using machine learning and high throughput screening to be able to predict exactly what kind of cage structure you need in order to get a specific substrate bound.’
References
A Ghosh et al, JACS, 2024, DOI: 10.1021/jacs.3c11005














No comments yet