An enzyme found in algae has been shown to efficiently convert medium-chain fatty acids – eight to 10 carbon atoms long – into medium-chain hydrocarbons similar to petrol. The work provides renewed hope for making green fuels from plant and algae-derived fatty acids.
In 2017, French researchers discovered fatty acid photodecarboxylase (FAP) in the green microalga Chlorella variabilis. FAP was found to be optimised to remove carboxyl groups from C16–C18 long-chain fatty acid substrates and produce hydrocarbons. As one of the few known natural photoenzymes that uses light to initiate biochemical reactions, its discovery triggered interest in its potential to produce environmentally-friendly, carbon-neutral hydrocarbons for fuels, chemicals and cosmetics.
Fatty acids are found in fats, oils and the membranes of all living cells. They’re made up of an aliphatic chain with a carboxyl group. ‘Decarboxylation of fatty acids using nothing but an enzyme and the energy of sunlight is a very elegant, simple and likely also the most environmentally friendly way of producing hydrocarbon fuels,’ explains FAP co-discoverer Pavel Müller at the University of Paris-Saclay, France.
Unlike fossil fuels, hydrocarbon fuels obtained by decarboxylation of fatty acids would be carbon neutral because the carbon dioxide they release would be offset by the biosynthesis of new fatty acids. However, subsequent studies suggested that FAP was unlikely to be an efficient catalyst for C5 to C12 short and medium chain fatty acids – the range found in liquid fuels. This was because these chains are not properly bound by FAP and tend to drift away from the active site, which leads to much lower quantum yields – a measure of how efficiently a process uses light.
Now, Muller and his colleagues have discovered an unexpected and previously unknown autocatalytic effect, which boosts the efficiency of FAP on medium-chain substrates. When the researchers used time-resolved spectroscopy to better understand the photodecarboxylation of medium-chain C7 to C12 fatty acids, they observed that initial alkane products appeared to promote the decarboxylation of further fatty acid substrates. This was most pronounced in C8 substrates.
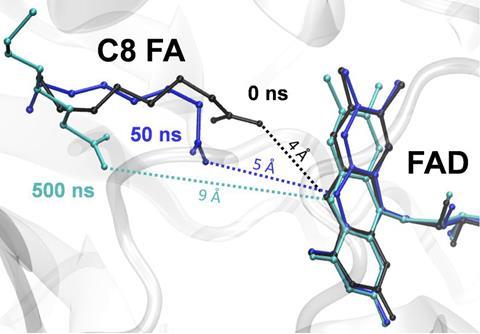
The alkane products seemed to help the shorter chains stay in place in the enzyme’s active site by filling the gap that would usually be filled by longer chains. This inspired further experiments that showed that alkanes can be added from the outset of the reaction to serve as co-catalysts, enabling decarboxylation quantum yields for medium-chain C7 to C12 fatty acids that were previously only achievable for long chain fatty acids.
‘As a result, this leads to comparable quantum yield for C8 and native C16 or C18 fatty acids,’ explains Müller. ‘Another surprising phenomenon was that the decarboxylation of the C8 substrate by FAP was not only as efficient as that of the C16 fatty acid, but an order of magnitude more efficient in vivo.’
‘The study demonstrates that the addition of small molecules can have a beneficial effect on catalysis when working with substrates that are likely smaller than those seen by the enzyme under physiological conditions,’ comments Nigel Scrutton who investigates enzyme catalysis at the University of Manchester, UK. ‘This is an interesting finding, however perceived benefits for the biosynthesis of hydrocarbons in biotechnology remain to be seen.’
‘Our ultimate goal is to design photostable FAP mutants that are highly efficient for short- and medium-chain fatty acids needed to produce hydrocarbon fuels,’ Müller says. However, he acknowledges that the performance and photostability of current bioengineered FAPs are not yet satisfactory. ‘We are planning to optimise the FAP enzyme through bioengineering to be efficient on medium-length substrates and hence suitable for use in bio-based production of gasoline-like liquid C5–C11 alkanes.’
References
PP Samire et al, Sci. Adv., 2023, 9, eadg3881 (DOI: 10.1126/sciadv.adg3881)









No comments yet