Unusual H-bond patterns revealed in single molecule image
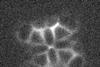
Hydrogen atoms in a molecule of cobalt phthalocyanine appear to be ‘shared’ between multiple centres

Hydrogen atoms in a molecule of cobalt phthalocyanine appear to be ‘shared’ between multiple centres