Researchers in Spain have devised a new parameter for comparing and understanding bonds. It describes the degree of interpenetration of the van der Waals crusts of two atoms, which they call the penetration index. It can replace previous distance measurements with simple calculations about crust overlap, and be applied to bonds ranging from strongly covalent multiple bonds to weak London dispersion forces.
‘The [van der Waals] crust is at a distance from the nucleus larger than the covalent radius but shorter than the van der Waals radius, it’s a region where a host of weakly attractive interactions occur,’ explains Santiago Álvarez, from the University of Barcelona, who developed the parameter alongside Jorge Echeverría from the University of Zaragoza.
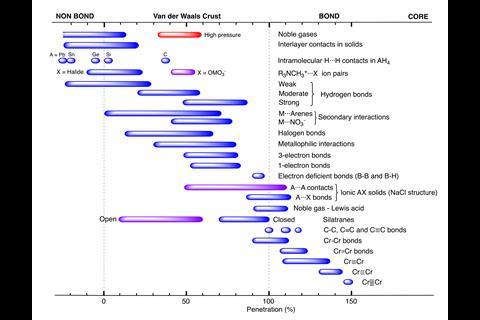
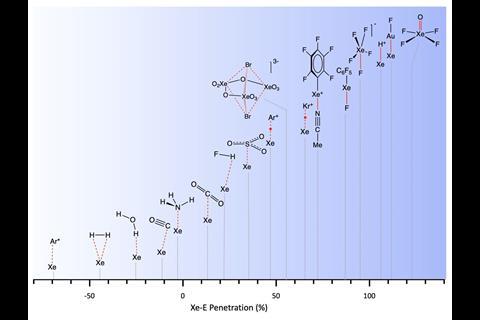
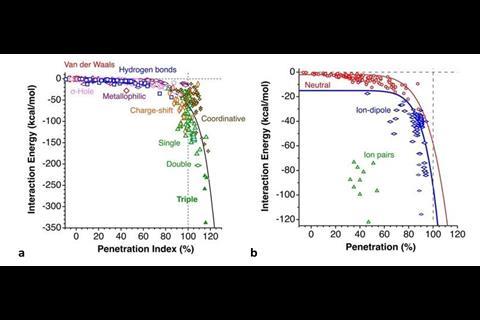
The duo rationalised that a penetration index based on the overlap between the van der Waals crusts of two atoms can describe all chemical bonds between atoms. A single covalent bond would have a value of 100% on the penetration index, with total overlap of the van der Waals crust. A traditional van der Waals force would have a value of 0%. There are instances of values outside this range, with double and triple bonds having values higher than 100%. Values in the negative range indicate interactions that occur outside of the van der Waals crust.
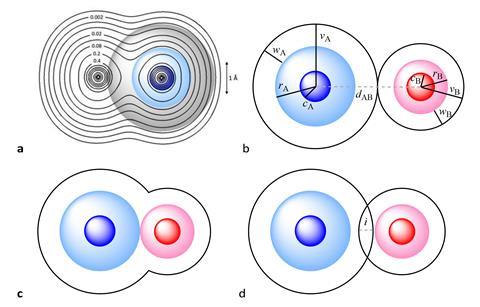
Álvarez says he and Echeverría decided to develop the system because ‘at the present time, we use bond distances to calibrate the degree of interaction of the atoms. But if you have two small atoms, they are of course [at a] much shorter distance than two heavier atoms with a similar bond … The purpose is to get a parameter that measures the degree of interaction between two atoms, which is independent of the size of the atoms.’ Álvarez goes on to explains that the overlap defined by the penetration index will be useful as it can give a quantitative meaning to all combinations of interatomic distances and ‘calibrate chemical bonding and structure across much wider sets of molecules’ than had previously been looked at.
A ‘herculean effort’
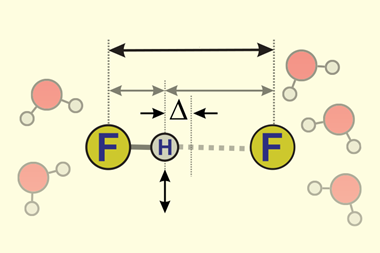
One area they calculated penetration indexes for was hydrogen bonding. Variations in the strength of hydrogen bond interactions correlated with penetration index values. Describing such bonding with a numerical value will allow chemists to be more precise than terms such as ‘weak’ and ‘strong’ hydrogen bonding. Examples of strong hydrogen bonding, such as those found in H–H+ and H2O+–H–OH2, both have penetration indexes above 50%. Weaker interactions were calculated to have extremely low and at times a negative penetration index.
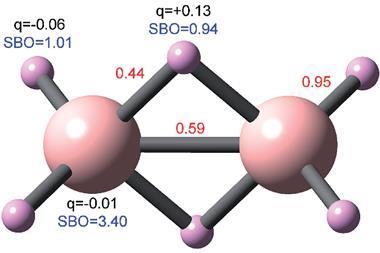
Other areas they analysed included arenes and cyclopentadiene bonding, and metals interactions between ions of the same sign in ionic solids. Alvarez says the method can be used in any area of chemistry where interatomic distances are relevant. He hopes it will ‘provide a better descriptor for the formation and dissociation of bonds in reactions’. He also expects the parameter will mean chemists rethink how they describe certain interactions, such as those found in frustrated Lewis pairs or adsorption mechanisms.
Martin Rahm, a theoretical chemist from Chalmers University of Technology in Sweden describes how Álvarez and Echeverría applied their quantitative penetration index to numerous areas of chemistry as a ‘herculean effort’. He adds that while the penetration index concept is simple, ‘simple methods can sometimes be very powerful ways to describe things’.
Thomas Manz, from New Mexico State University in the US, is also a theoretical chemist. He says the penetration index is a ‘useful tool to quantitatively interpret diverse chemical bonds’ but says the work has some fundamental limitations. ‘Because this penetration index is based on a chemical element’s covalent radius and van der Waals radius, this penetration index does not strongly correlate with the electron density overlaps between atoms in ionic crystals.’ Manz says this is because the index is based on covalent radii rather than ionic radii.
References
This article is open access
S Álvarez and J Echeverría, Chem. Sci., 2023, DOI: 10.1039/d3sc02238b














No comments yet