Transition-metal-like complexes with a Be(0) centre have been synthesised for the first time
The area of multiple bonding and zero-valent ‘molecular element’ chemistry, written off for many years as particularly unexciting in terms of covalent bonding, has now been opened up by researchers in Germany.
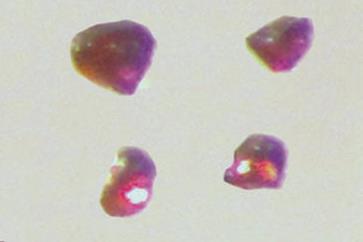
Holger Braunschweig and colleagues at the Julius Maximilian University of Würzburg have made beryllium complexes they say are the first stable and neutral compounds containing a zero-valent s-block metal.
S-block metals — such as sodium, potassium and beryllium — are strong electron donors, readily shedding their negativity and forming positive ions. But if they can be induced to sit comfortably in a molecule in the zero- or low-valent (less than fully oxidised) state, they can adopt some of the properties of their transition metal cousins.
While such complexes have been the target of synthetic research for a decade or so, and so-called alkalide salts have been claimed wherein the metal ion is in the –1 formal oxidation state, the zero-valent state has existed only in the computational world, where calculations have hinted at a possible future for Be0 complexes. Theoretical work has previously suggested that N-heterocyclic carbenes (NHCs) would be the way forward in making Be+1 and Be0 complexes, although none had been synthesised in anything other than the +2 oxidation state.
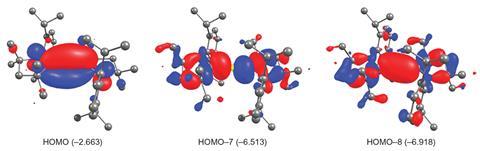
Braunschweig’s group worked with these computational concepts but reasoned that the limited success with NHCs suggested an alternative pi-bonding ligand was needed. Given that cyclic (alkyl)(amino)carbene (CAAC) ligands have been used to stabilise unusual transition metal and p-block compounds, the team thought they might obtain a similar stabilisation in a beryllium complex. The complexes they synthesised contain very short bonds between the beryllium and carbon atoms, and linear beryllium coordination geometries are observed reinforcing the idea of strong multiple Be–C bonds.
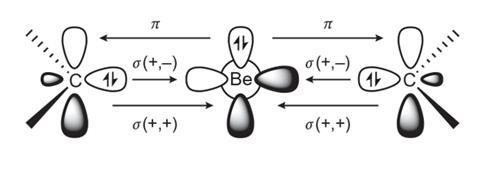
The compounds are also highly coloured, a property that in the world of metals is almost uniquely the reserve of the transition block and not usually observed in compounds of s-block metals. Their structural, spectroscopic and theoretical data show that the complexes have a closed-shell singlet configuration that has a zero-valent Be0 metal centre. Additionally, an unusually strong three-centre two-electron pi bond across the C–Be–C group endows the complexes with surprising stability.
‘This is a remarkable achievement, which demonstrates again the advantage of cyclic (alkyl)(amino)carbenes (CAACs) over NHCs for stabilizing highly reactive intermediates,’ comments CAAC discoverer Guy Bertrand of the University of California, San Diego, US, who was not involved in the study.
Michael Hill from the University of Bath, UK, also describes the work as ‘truly impressive’. ‘This compound…is sure to provoke renewed interest in beryllium chemistry. Given the unusual bonding within this species, I’m looking forward to seeing further reports on its reactivity and chemical behaviour,’ he says, although he adds that because of the element’s toxicity, ‘it doesn’t seem likely that molecular beryllium species will find a real world application any time soon’.
References
M Arrowsmith et al, Nat. Chem., 2016; DOI: 10.1038/nchem.2542
![Frontier orbitals of [IrO4]+ (left), which scientists recently synthesised, and predicted [PtO4 ]2+ (right)](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/5/1/1/101511_oxidation-state-10-exists_angewandte_chemie_630m.jpg)






No comments yet