New material has platinum in -2 oxidation state
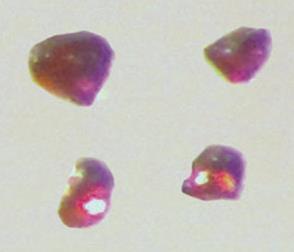
The first intermetallic double salt featuring negative platinum ions has been isolated by scientists in the US.
The caesium platinide hydride compound was discovered by materials researchers Volodymyr Smetana and Anya-Verena Mudring at the US Department of Energy’s Ames Laboratory in Iowa. It is the first compound, composed of three elements, to contain platinum in the –2 oxidation state.
While other alkali-metal platinum hydrides are known, they are coordination compounds involving positively charged platinum species. Examples of chemicals containing truly negatively charged metal ions are rare.
The new material was isolated as a cherry-red crystalline solid, following the reaction of platinum with caesium metal and caesium hydride. Nuclear magnetic resonance, crystallographic experiments and computational studies confirmed the structure of the compound.






![Frontier orbitals of [IrO4]+ (left), which scientists recently synthesised, and predicted [PtO4 ]2+ (right)](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/5/1/1/101511_oxidation-state-10-exists_angewandte_chemie_630m.jpg)








No comments yet