Recently discovered [Sb3Au3Sb3]3– has multiple groups hunting for answers
In the wake of the first experimentally isolated all-metal sandwich complex last year,1 two independent groups from China have put forward their theoretical take on this inorganic rarity.2,3
Sandwich complexes are a class of inorganic compounds, typified by ferrocene. They feature a metal ion sandwiched between two aromatic ligands, which are bound to the central metal through haptic covalent bonds. Structurally similar species to ferrocene, with different metals or coordinating ligands, are generally termed metallocenes.
Ferrocene’s discovery in 1950, and confirmation of its unusual bonding, piqued the community’s interest with the advent of other metallocenes, carbon-free ‘sandwiches’ and cores with multiple metal atoms. However, exclusively metal sandwich complexes have remained elusive, persisting as nothing more than theoretical pipe dreams, until recently. Last year, a team led by Zhong-Ming Sun of the Chinese Academy of Sciences in Changchun, China, isolated [Sb3Au3Sb3]3–, a layer of three gold atoms wedged between two aromatic antimony sheets, the first of its kind.1
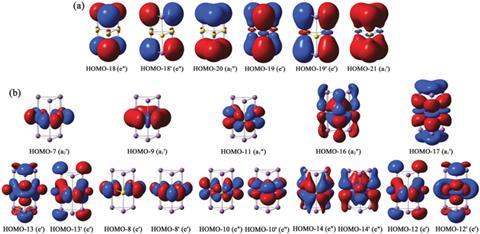
Finding such a system has prompted theoreticians to seek answers. Hua-Jin Zhai of Shanxi University led one of these contingents: ‘Not many people, even professional physical chemists, can correctly understand the nature of bonding in such nanosystems.’ Using a variety of computational methods, his team has shown that nearly 20 molecular orbitals contribute to the electronic glue holding the layers together. The collective result can be generalised as three Sb–Au–Sb three-center-two-electron bonds – a similar bonding model to diborane. However, unlike diborane, ‘none of these molecular orbitals are primarily responsible for the interlayer bonding’, says Zhai. This indicates that the overall bonding picture for [Sb3Au3Sb3]3– is more complicated than theory allows.
Zhai’s work follows a parallel study by a group based in Tsinghua University headed up by Jun Li.3 By looking at [Sb3Au3Sb3]3– and its analogues, Li’s group postulated that other viable oligomers await discovery. Their work is similar in its approach and overall conclusions, though the studies are at odds over how they interpret aromaticity in the complex. Zhai is unconvinced by Li’s findings: ‘A triangular, six s-electron system is equivalent to three two-center-two-electron s-bonds, which is a localised system and hence not aromatic. This is conceptually wrong.’
Pablo Denis, a theoretical chemist from the University of the Republic, Uruguay, describes the bonding in the complex as fascinating. ‘It is interesting that oligomers [in Li’s study] are stable’, he says. ‘The chemical bonding is very complex … [though] the big picture is not too different as both studies come to the conclusion of three-center-two-electron bonding.’ John Slattery, an experimental and computational chemist at the University of York, UK is also excited by [Sb3Au3Sb3]3–. ‘It’s great to see some detailed theoretical investigations into the structure and bonding of this ion,’ he remarks. ‘Bonding in these clusters is not simple to describe and the results of these DFT studies provide some very interesting insights and I’m sure [they] will prompt significant discussion.’
Given the infancy of this peculiar system, it is likely that more studies of this nature will emerge, if not synonymous complexes. It remains to be seen how theoreticians will respond to the nuanced differences of the two studies, as small discrepancies between models can ultimately amount to disparate conclusions. ‘These are the types of molecules where the discussion of structure and bonding can create significant debate over a number of years,’ cautions Slattery. ‘I think this could be the start of the story with this ion, rather than the end of it.’
References
1 F-X Pan et al, J. Am. Chem. Soc., 2015, 137, 10954 (DOI: 10.1021/jacs.5b07730)
The following articles are available for free until 21 June 2016
2 X-R You et al, Phys. Chem. Chem. Phys., 2016, DOI: 10.1039/c6cp00101g
3 W-L Li et al, Dalton Trans., 2016, DOI: 10.1039/c6dt00602g






No comments yet