Prediction suggests platinum oxide compound is stable in +10 state for nearly a year
Two scientists in the US have predicted that the +10 oxidation state exists.
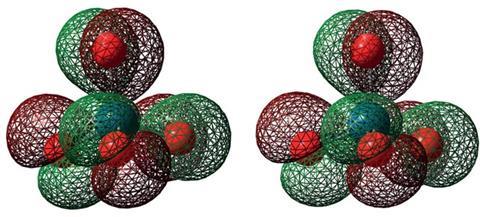
Up until 2010, it was assumed an atom’s oxidation number in a compound could only range between -4 and +8. But an international group of scientists predicted the +9 oxidation state could exist in an iridium oxide cation, [IrO4]+. A team in China confirmed this prediction in 2014, forming [IrO4]+ via the pulsed-laser vaporisation of iridium.
Now, Haoyu Yu and Donald Truhlar, both based at the University of Minnesota, have calculated the +10 state will exist in the platinum oxide, PtO42+. Using density functional theory, the team looked at the enthalpy profiles and atomic charges of several platinum and palladium compounds.
PtO42+ is the most stable in the +10 state with a lifetime of 313 days. The team also noted the compound shares a similar electronic structure to [IrO4]+.
References
H Yu and D Truhlar, Angew. Chem. Int. Ed., 2016, DOI: 10.1002/anie.201604670









1 Reader's comment