Could MOFs add shock-absorber to their list of potential applications?
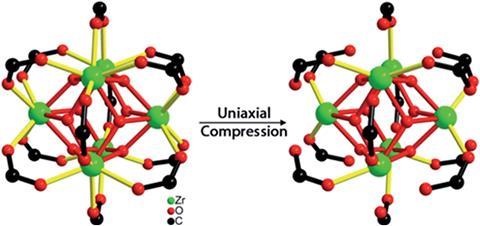
Scientists in the US have found that a metal–organic framework (MOF) known for its robustness takes in the same amount of energy as a TNT blast releases when it breaks.
MOF materials are porous framework solids whose typical applications include gas storage, separation and catalysis. Scientists have studied the zirconium-based MOF, UiO-66, in more detail than most. It’s easily synthesised, has a well-known structure and is strong. Unlike some other MOFs, it doesn’t react with water, and on removing its residual solvent, the framework remains intact with true, empty voids.
Intrigued by its properties, a research team led by Kenneth Suslick at the University of Illinois at Urbana-Champaign investigated what happened if they crushed UiO-66. Using flat punch nanocompression, Suslick’s team placed UiO-66 on a silicon wedge holder and squashed it using a diamond punch with pressures of up to 10GPa. They found that the MOF absorbed up to 4kJg–1 of mechanical energy, which is comparable to the energy that explosives such as TNT (2,4,6-trinitrotoluene) release when they explode.
‘When we crush MOFs under pressure, the structure collapses and breaks some of the chemical bonds that hold the structure together. Breaking chemical bonds costs energy and a lot of it,’ explains Suslick. ‘The idea that crushing a MOF requires, gram for gram, as much energy to be taken up as an explosive gives off is a remarkable observation. And, we were actually able to determine which bonds break and how that changes the structure.’ This type of observation is uncommon.
‘The connection between the mechanical world and the chemical world has rarely been studied and is not well understood,’ says Suslick. So, to better understand its properties, his team used synchrotron x-rays and infrared spectroscopy to detect and monitor bond breakages and changes in UiO-66’s chemical structure. Compressing the MOF at 1.9GPa broke over half of the Zr–O bonds between Zr(IV) ions and carboxylate groups in UiO-66. They also studied changes in the vibrations of atomic bonds to pinpoint what happens to the relationship between mechanical properties and chemical structure when they compressed the MOF at different pressure.
Understanding how UiO-66 behaves under these conditions may lead to potential new applications. MOF expert Jeffrey Long from the University of California, Berkeley, thinks so. ‘This is a creative piece of research that nicely demonstrates an intriguing new potential application of MOFs: the rapid dissipation of mechanical energy,’ he says.
As well as chemical bonds, structural defects determine the strength of the UiO-66 framework. By tweaking the number of defects in the system, the team suggest it may be possible to exploit UiO-66’s newly discovered properties to create a tailored shock absorber MOF that collapses at a higher or lower pressure. The researchers have yet to fully explore this possibility but have further tests in the pipeline.








No comments yet