Dispelling nickel’s catalytic demons
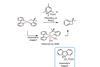
Stable Ni(IV) complexes could help nickel rival its flashier platinum-group cousins, says Karl Collins

Stable Ni(IV) complexes could help nickel rival its flashier platinum-group cousins, says Karl Collins