Sometimes unnatural molecules can be more challenging to synthesise than natural metabolites
For some, the phrase ‘total synthesis’ conjures images of Robert Woodward lighting another cigarette as he works into the evening on his groundbreaking preparation of vitamin B12. For others, KC Nicolaou’s searing yellow structures of polycylic brevetoxins on infinitely black slides are a most vivid image, while for a younger generation, the term is inherently linked to Phil Baran’s eternal suntan and strategic masterclasses.
Total synthesis for most organic chemists is intrinsically bound to natural products, even though synthesis of unnatural molecules is fundamental to advancing a plethora of fields – for example, the molecular machines that won last year’s Nobel prize in chemistry. These molecules require multistep syntheses, equally as complex as a given natural product.
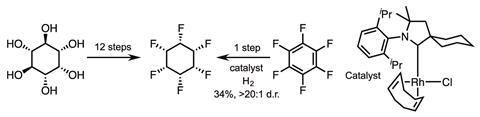
One unnatural target that had long been pursued, due to its expected physical properties, is all-cis 1,2,3,4,5,6-hexafluorocyclohexane – a facially polarised cyclohexane now known to be one of the most polar organic molecules in existence – exquisitely prepared in 12 steps by David O’Hagan and his team at the University of St Andrews, UK, in 2015.1 As with all great total syntheses, this achievement was the culmination of several years of effort and will, pleasingly for the team, always be honoured as the first synthesis. According to the adage, we will only remember the first and the best; and whilst the first is clearly marked, the best may forever be just around the corner. That said, the second total synthesis of all-cis 1,2,3,4,5,6-hexafluorocyclohexane – in a single step from hexafluorobenzene – is likely to hold the title of ‘best’ for some time!
Frank Glorius and his team at the University of Münster, Germany, identified a rhodium catalyst with a cyclic (alkyl)(amino)carbene (CAAC) ligand that enables stereoselective hydrogenation of fluorinated arenes.2 This may seem somewhat trivial, but until now, viable methods for preparing fluorinated cyclohexanes – particularly with cis conformation – were essentially unknown. The ease of hydro-defluorination has hampered development of hydrogenation methods (figure 1).
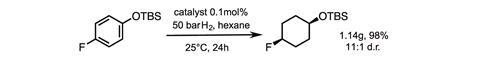
Glorius and his team began by testing how the Rh-CAAC catalyst reduces (tert-butyldimethylsilyloxy)-4-fluorobenzene. In trifluoroethanol, the major product is the hydrodefluorinated cyclohexane, with only 17% of the target compound. Switching solvents to hexane, or diethyl ether where a more polar solvent is needed, improves yield and stereoselectivity. Tweaking the hydrogen pressure and catalyst loading allowed the team to isolate the fluorinated cyclohexane product in 93% yield with an excellent diastereomeric ratio of 13:1.
Hydrogenation can be a fickle beast, and stereoselectivity is prone to unpredictable variability, particularly when heteroatoms are added to the substrate. This reaction shows excellent tolerance to some of the most synthetically useful functional groups, including protected amines, esters, silylated alcohols and even boronic esters. In total, the Münster team prepared 15 new functionalised fluorinated cyclohexane building blocks with remarkable diastereoselectivity, and demonstrated several gram-scale reactions.
From a more fundamental perspective, the team systematically hydrogenated a number of mono-and multi-fluorinated benzenes, as well as biaryl structures and naphthalenes. As prescribed by the unwritten rules of methodology papers, Glorius and the team used their new method to synthesise building blocks for pharmaceutical product analogues, as well as a unique N-heterocyclic carbene ligand and two liquid crystal analogues.
This work opens up rapid access to a multitude of functionalised building blocks with excellent stereocontrol, as well as streamlining syntheses of known compounds. As always, it would be useful to know the limitations of the reaction, particularly with respect to functional groups that are not tolerated. Like all greedy children, I simply want more – and a single-step synthesis of fluorinated piperidine derivatives is at the top of my list.
Whilst Glorius and his team were not the first, the single step synthesis of cis-1,2,3,4,5,6-hexafluorocyclohexane from cheap and commercially available starting materials is hard to beat, and could secure them a place in the annals of ‘unnatural’ product synthesis for some time to come.
References
1 N S Keddie et al, Nat. Chem., 2015, 7, 483 (DOI: 10.1038/nchem.2232)
2 M P Wiesenfeldt et al, Science, 2017, 357, 908 (DOI: 10.1126/science.aao0270)


![[Br4F21]- index image](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/1/7/3/532173_br4f21indeximage_49094.jpg)








1 Reader's comment