Photochemical reactions have been happening for as long as light and matter have existed. And while chemists have been studying these reactions in earnest since the 19th century, it’s only more recently that they’ve been making the most of them. Today, using light as an energy source for chemical reactions has never been so vital.
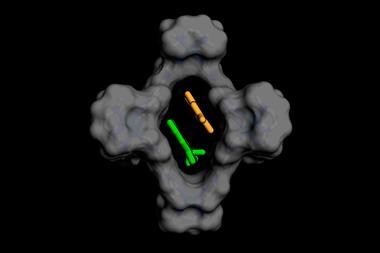
Several stories we’ve covered recently convey the kaleidoscope of developments in photochemistry. A few weeks ago we reported on a laser-based process that makes ammonia under ambient conditions. And just before that we wrote about a system that converts light into chemical energy, in the form of out-of-equilibrium photostationary states. It combines a macrocyclic host and a photosensitiser, and was used to transform the stable E isomer of azobenzene to the metastable Z isomer. Confining the process to a host molecule means that when azobenzene photoisomerises into the Z isomer, it no longer fits in the cavity so gets pushed out. Plus, the process uses visible light, which is preferable to previously used ultraviolet light.
Moreover, quantum dots – the tiny crystals honoured with this year’s chemistry Nobel prize – have also found use as photosensitisers and photocatalysts. Light irradiation equal to or greater than a quantum dot’s band gap can excite its valence band electrons to the conduction band, creating electron–hole pairs. Those electrons can be used for splitting water and reducing carbon dioxide, and the holes can degrade organic pollutants. And as well as their tuneable redox potential, quantum dots’ colloidal nature supports interactions between them and their substrates, and also makes them easy to remove and recycle, echoing the benefits of both homogeneous and heterogeneous catalysis.
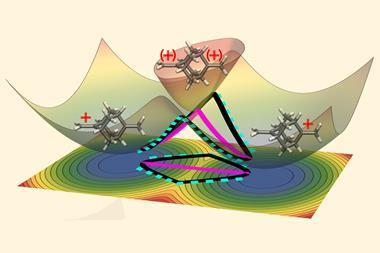
Of course, theory relating to the world of photochemistry has been progressing too. Right at the beginning of October, we described how researchers have extended instanton theory to compute quantum tunnelling rates through conical intersections. Originally just conjecture, we are now coming to realise that conical intersections – which are molecular geometries where two electronic energy surfaces have the same energy – are ubiquitous in molecular systems and can be remarkably efficient at conferring them with photoreactivity or photostability.
Such an abundance of developments on both experimental and theoretical fronts indicates that photochemistry is set for a bright future.













No comments yet