A new supramolecular capsule helps trigger the isomerisation of azobenzene with visible light. For the first time, the system enables full conversion to the metastable Z form, unlocking new possibilities for photoswitches, including for applications in energy storage and non-invasive medical treatments.
Azobenzenes are the most popular photoswitches – molecules that change shape upon exposure to light. Usually, ultraviolet light triggers the transformation between the stable E and metastable Z isomers, but this reaction faces several hurdles, including problems associated with selectivity and efficiency. ‘The core problem for azobenzenes is E to Z isomerisation, because the E [form] gets activated at wavelengths that activate the Z form,’ explains Nathalie Katsonis, an expert in photoswitches and supramolecular chemistry at the University of Groningen, in the Netherlands, who wasn’t involved in the work. ‘It’s a super original [study] that proposes a concept to overcome this limitation,’ she adds.
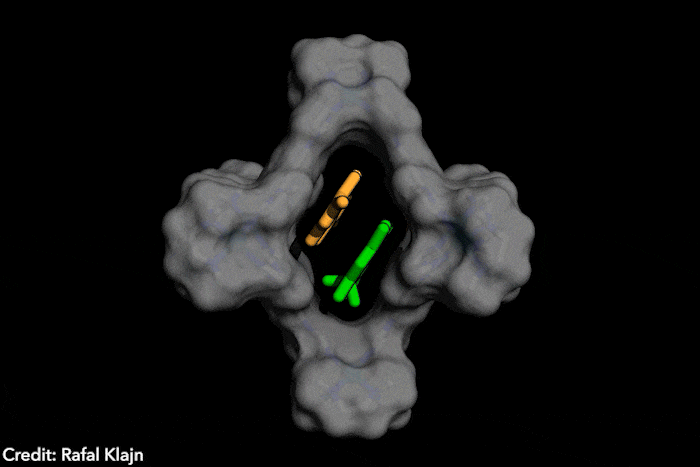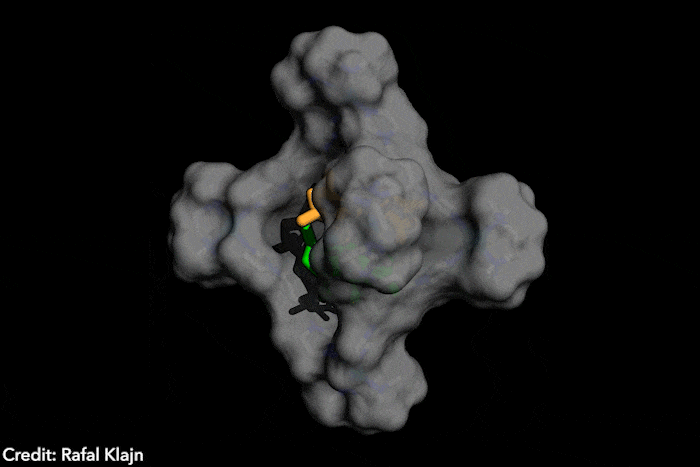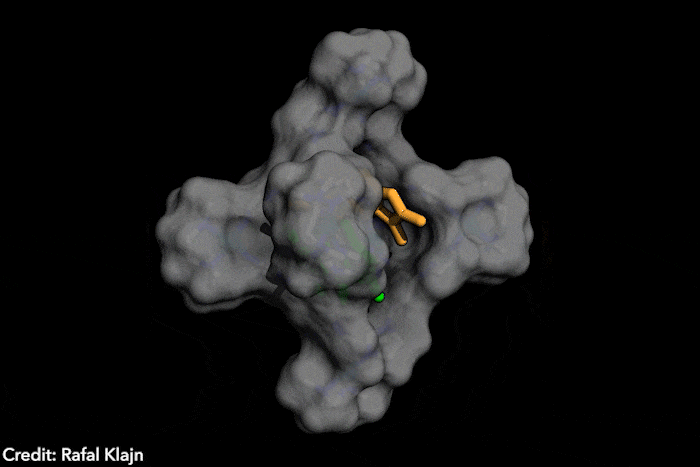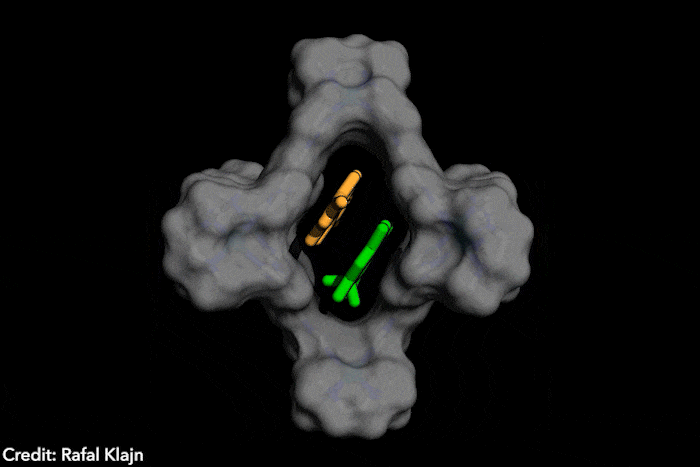
The idea – named disequilibration by sensitisation under confinement – combines two simultaneous strategies, both based on encapsulating the azobenzenes inside a supramolecular structure. The capsule contains both the azobenzene and a photosensitiser. The latter gets excited under exposure to visible light, which wouldn’t naturally trigger a conformational change in the photoswitch. In close confinement, the photosensitiser transfers the energy to the azobenzene, causing the conversion from the E structure to the Z form, which subsequently leaves the supramolecular cage, displacing the equilibrium instead of reverting to the most stable isomer. ‘The excitation of the switch only occurs by energy transfer [from] a photosensitizer, […] the system is excited at energies where neither E or Z can actually absorb light,’ explains Katsonis. Therefore, you [only] get efficient energy transfer for the E form, which fits in the cage’ she adds. Because the Z form leaves the cage, the reverse reaction is prevented.
‘This work brings synthetic systems closer to the efficiency and elegance [of] enzymes,’ says project leader Rafal Klajn, from the Institute of Science and Technology in Klosterneuburg, Austria. ‘[Our] synthetic molecule exhibits characteristics typically associated with enzymes – it’s soluble in water but has a hydrophobic cavity capable of binding organic molecules and converting them into other molecules, which do not fit […] and are expelled once formed,’ he adds. This chemical conversion, induced by a photosensitive dye that captures visible light reminds researchers of enzyme cofactors, such as the polyporphiryns in haemoglobin and chlorophyll.
The ‘closed cage’ conversion of photoswitches also brings advantages in terms of selectivity. Because the supramolecular structure is positively charged, it’s predisposed to trapping and transforming negatively charged azobenzenes. ‘Such selective photoswitching would be very hard [with] the traditional method, [which] exposes a solution of unconfined azobenzenes to light,’ says Klajn. In this case, all the dissolved molecules switch unselectively, he explains.
‘[In a] mixture of positively and negatively charged azobenzenes, […] only the negatively charged are converted,’ adds Kasper Moth-Poulsen, an expert in photoswitches systems and smart materials based at the Polytechnic University of Catalonia in Barcelona, Spain. ‘This [experiment] confirms the mechanism, but also illustrates how the host [could] be used as a supramolecular filter to only convert a certain type of photoswitch,’ he adds.
Additionally, ‘[this] supramolecular solution allows the use of less harmful light to photoisomerise azobenzene, as well as fully convert it from E to Z,’ explains Igor Schapiro from the Hebrew University of Jerusalem, Israel, who co-led the study. ‘Both properties were highly sought after in the field, and only became available now.’
The activation of azobenzene photoswitches with visible wavelengths, instead of ultraviolet light, could enable interesting biomedical applications. ‘The use of red light is crucial for the pharma industry, because red light penetrates through the skin, [which allows] triggering of the photoswitches in a non-invasive manner,’ adds Schapiro.
‘Ultraviolet light is […] generally harmful to soft molecular materials, as it generates highly reactive radical species,’ says Katsonis. Visible light isn’t harmful and its penetration in skin is substantially larger – it seeps deeper, approximately an order of magnitude, she adds.
‘It’s [usually] a challenge is to reach photoconversion inside the body, […] with advanced methods like this one, new ways of controlling drug activity may become available,’ says Moth-Poulsen.
Looking to other applications, Moth-Pulsen points out that the system could also bring benefits for energy storage. ‘In solar energy storage, using a larger part of the solar spectrum for photoconversion is [still] a challenge, [and] this approach extends the useable wavelengths,’ he says.
Another exciting element in this discovery ‘is that several molecular combinations seem to work’ says Moth-Poulsen, who notes that the supramolecular system was demonstrated with nine azoarene switches and four photosensitisers.
‘We envision the methodology [could] be extended to other classes of photoswitches, including spiropyrans and indigos,’ adds Klajn. ‘We’ve now shown a few other sensitizers and azoarenes switch efficiently, [even when] attached to polymer chains,’ he says. ’The methodology is general and […] expanding rapidly.’
References
J Gemen et al. Science 2023, 381, 6664, 1357, DOI: 10.1126/science.adh9059
















No comments yet