Organic wires conduct electrons 800 times faster than other molecular counterparts by letting them hitch a ride on a vibrational wave
Researchers in Germany and Japan have shown that a new type of organic molecular wire – which is flat and rigid – can transfer electrons at more than 800 times the speed of its conventional, flexible counterpart. Unlike many molecular wires, whose properties are often investigated at low temperatures and in vacuum, the new study was carried out at room temperature and in solution, giving it, say the researchers, greater potential for applications in molecular electronic devices.
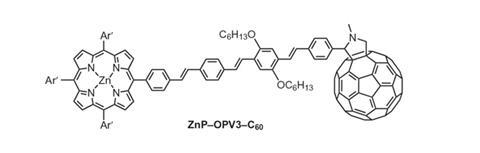
Typically, organic molecular wires consist of linear molecules containing alternating single and double bonds. The resulting p-conjugation provides the conditions for electron tunnelling along the molecule, the most important electron transfer mechanism. However, the single bonds provide a point of rotation and flexion, which disrupts the electronic coupling along the wire and reduces the efficiency of electron transfer.
To tackle the issue of molecular flexibility, Dirk Guldi, of the Friedrich-Alexander University Erlangen-Nürnberg, and colleagues investigated electron transfer in molecular wires based on carbon-bridged oligo-p-phenylenevinylenes (COPVs). Here, the single bonds are replaced by ring structures, conferring rigidity and restricting rotation, but retaining the vital p-conjugation for a high degree of electronic coupling.
The team compared electron transfer rates in COPVs with those in their non-bridged counterparts, by attaching a photo-excitable electron donor at one end, zinc porphyrin, and an electron acceptor, fullerene, at the other. The researchers showed that the electron transfer rate was 840 times higher in the system using the rigid molecular bridge than for the flexible one.
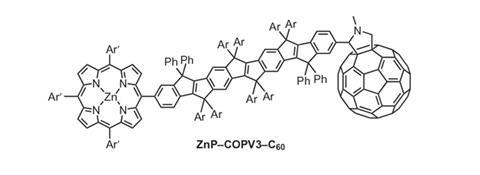
Analysis suggests that around 120-fold of this increased rate is down to the better coupling between the donor and acceptor. But most of the enhanced efficiency could be due to electrons riding a wave of vibrational energy along the molecule, Guldi suggests. As electrons tunnel along the bridge, vibrational energy is induced and this can increase the speed of transfer. In flexible molecular wires these vibrations are quickly dampened, so the effect is lost. By contrast, in the flat, rigid COPVs the vibrations are much more long-lived.
‘I think this finding is important because we may be able to use these rigid bridges in molecular electronic applications to get maximum speed of electrons at room temperature,’ Guldi says.
Commenting on the work, Robert Metzger, a molecular electronics expert from the University of Alabama in the US, says that the researchers ‘have made some new “monster molecules” that show rather convincingly that a rigid, and inflexible linkage enhances considerably the speed of charge separation; this is a significant result’.










No comments yet