A shape-shifting nanomedicine that exploits subtle differences in the cellular environment can selectively target and penetrate cancerous cells. The cofactor-assisted complex exists as stable nanofibres in the blood but assembles into virus-like clusters in the acidic conditions surrounding tumours. Once inside the tumour cells, the assembly rearranges again, releasing its drug cargo and inducing cell death with minimal side effects.
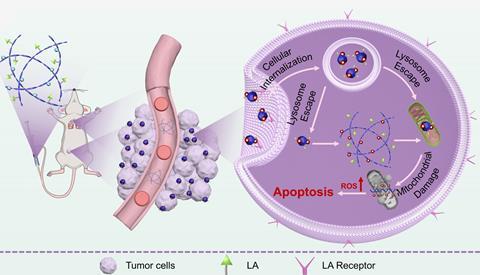
The last 10 years have witnessed staggering advances in cancer therapies, but the similarity of patients’ own tissues to tumour cells means many of these treatments are accompanied by devastating side effects. Efforts to develop more targeted medications increasingly leverage small differences in the localised tumour environment, such as increased acidity or hypoxia, and have inspired a new generation of stimuli-responsive treatments. In particular, self-assembling nanomedicines – in which the active unit only forms in the vicinity of cancer cells – have proven a promising precision approach, but the challenge of designing and preparing these complex systems means this treatment strategy is still in its infancy.
Inspired by the way molecular chaperones regulate the folding of other cellular proteins, Jing Sun and her team at Jilin University in China developed a simple synthetic protein-like cofactor to act as a pH-responsive delivery vehicle for the potent anticancer terpenoid ursolic acid (UA). ‘The polypeptoids mimic natural proteins but are more flexible, allowing controlled self-assembly of UA,’ explains Sun. ‘The polypeptoids are modified with DMMA, a pH-sensitive molecule that enables long circulation in blood but makes them positively charged in acidic tumours for better uptake. Additionally, lactobionic acid is incorporated as a targeting ligand to bind overexpressed receptors on cancer cells.’
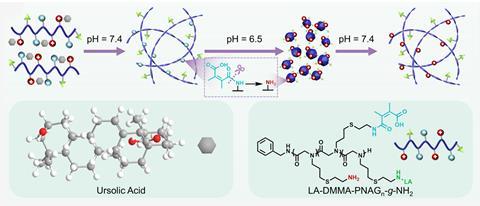
Under the neutral conditions of the bloodstream, insoluble ursolic acid assembles into stable nanofibres with the polypeptoid cofactor, aggregating into virus-like clusters in the acidic tumour environment. These spherical particles are significantly better at penetrating tumour cells, boosting the bioavailability of the ursolic acid and limiting the uptake of the drug in healthy tissue. ‘Once inside the cell, the elevated oxidative stress characteristic of the cytoplasm triggers the cleavage of thioether bonds within the structure, resulting in the release of UA and polypeptoids,’ Sun explains. ‘These components then accumulate around mitochondria, damaging this critical organelle and triggering cancer cell death.’
Intriguingly, a proportion of the nanocomplex also reassembles into nanofibers within the cytoplasm, retaining a supply of the drug within the cell environment. For Jiban Jyoti Panda, a nano-biotechnologist at the Institute of Nano Science and Technology in India, this reversible transformation was a standout strength of the work, which would crucially facilitate sustained drug release and reduced dosing frequency.
This pH-responsive mechanism of action was then validated in a mouse model, with the combined polypeptoid-ursolic acid assembly showing a significantly enhanced therapeutic effect over the individual components and reducing both tumour growth and metastasis. The condition-specific activation of the treatment results in an excellent safety profile and the same design could be broadly applicable to any solid tumours exhibiting acidic microenvironments or oxidative stress, says Sun.
The team’s careful cofactor design impressed Panda, who highlights the easy synthesis and synergistic action as other notable strengths of the work. ‘This research presents a progressive approach towards developing biologically compliant in-situ assembled structures for cancer-targeted drug delivery,’ she says. ‘More extensive pharmacodynamic investigations are necessary to establish its fate in the body [and] the route of clearance and formation of byproducts is also an important aspect to understand the long-term toxicity of the nanocomplex.’
Further work is clearly required to advance this nanocomplex towards the clinic but for now, Sun’s team are focusing on applying a similar polypeptoid self-assembly approach to other challenging disease targets. ‘This carrier-free strategy simplifies formulation and has potential for scalable production,’ says Sun. ‘[We] will focus on the molecular design of polypeptoids to enhance their self-assembly properties and therapeutic effects, while also exploring their potential with other natural plant compounds beyond ursolic acid.’
References
M Lin et al, J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c01214














1 Reader's comment