Work demonstrated on Bergman cyclisation offers route to valuable new reactions
A single molecule chemical reaction has been switched back and forth by researchers in Switzerland. Using voltage pulses applied to the needle tip of a scanning tunnelling microscope (STM), Leo Gross and his co-workers at the IBM Research Centre in Ruschlikon, near Zurich, could break open a carbon–carbon bond in a molecule containing a chain of three carbon rings, converting two of the rings into one large one. With another pulse, they could ‘pinch’ the large ring back into two.1
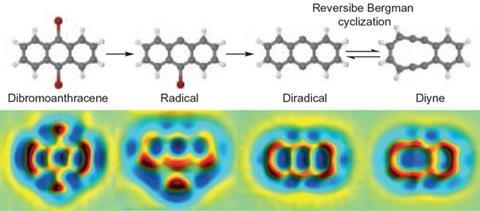
The reaction, known as a Bergman cyclisation after its discovery by Robert Bergman and Richard Jones in 1972, is important in the action of so-called enediyne anticancer drugs, which can cleave DNA. Enediynes contain two carbon–carbon triple bonds joined by a double bond. This constitutes a reactive ‘warhead’ in the drug. The triple bonds can crosslink to form a ring with two unpaired electrons: a diradical that then attacks the DNA backbone.
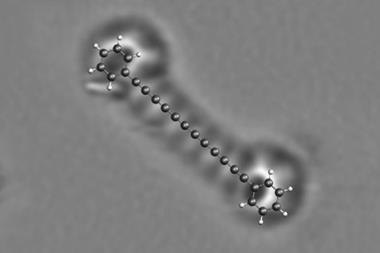
Having previously used scanning probe microscopes to produce and image reactive intermediates rather like these on a solid surface2, Gross and colleagues wondered if they might extend the same methods to gain control over a Bergman cyclisation. They decided to study an enediyne in which the two triple-bond arms were already linked end to end via a four-carbon bridge, making a 10 carbon strained ring. This can be interconverted, via the Bergman cyclisation, with a molecule comprised of three conjoined six-membered rings: a diradical version of anthracene.
Reversing a reaction
The researchers carried out the reaction in reverse: first they made the anthracene diradical, and then they converted it to the cyclic diyne. They began with dibromoanthracene, adsorbed on sodium chloride. With two voltage pulses applied to an STM tip held just over the molecule, they could cut off each bromine atom in turn, creating the diradical. A further voltage pulse then cut open one side of the middle ring, creating the 10-membered diyne.
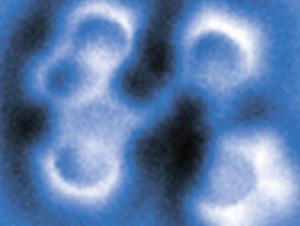
At each step, Gross and colleagues could inspect what they had made using an atomic force microscope (AFM). With a carbon monoxide molecule attached to the AFM tip, the IBM team has previously shown that molecular structures like these can be imaged in extraordinary detail, the atomic framework visible almost like a ball-and-stick model. They verify what the images show by comparing them with theoretical calculations for the respective structures.
Could the reaction be switched in either direction by voltage pulses? To study that, Gross and colleagues first lodged the diyne molecule at a step edge on the salt surface, so that it wouldn’t skitter out of sight when switched. They found that with pulses of 1.64V, they could convert it into the anthracene diradical, and then open up the other side of the middle ring to create the other isomer (strictly, a topomer) of the diyne.
Expanding horizons
This could be a great new way to find useful reactions, says Gross’s coauthor Diego Peña of the University of Santiago de Compostella in Spain, who supplied the chemical expertise for the study. You simply apply a pulse to an adsorbed molecule and see if and how it has changed. ‘In solution chemistry, after many decades of rigorous research the chances of finding new reactions are quite limited,’ he says. ‘On the contrary, tip manipulation of molecules is in its infancy, and I would bet all my savings on its future.’ The method might also be used to explore reaction mechanisms, perhaps paving the way to improved yields. Gross sees a more distant goal too, towards which this is just a very first step. ‘What we intend in the long run is the fabrication of devices made from few molecules on a surface, assembled by atomic manipulation,’ he says.
‘This is a beautiful example of what non-contact AFM has made possible as a technique,’ says Felix Fischer of the University of California at Berkeley, US, – not least because it makes chemical transformation so directly visible. ‘I am absolutely sure that new textbooks in organic chemistry will try to secure the copyrights to this image, and that it will be taught by this very visual example for generations of undergraduates to come’, he says.
Another chemist delighted to see the result is Bergman himself, also at Berkeley. ‘When we first reported this reaction I had no idea that it would be biologically relevant, or that the reaction could someday be visualised at the molecular level,’ he says.
Correction: The headline for the article was changed on 26 January 2016
References
1 B Schuler et al, Nat. Chem., 2016, DOI: 10.1038/nchem.2438
2 N Pavlicek et al, Nat. Chem., 2015, 7, 623 (DOI: 10.1038/nchem.2300)









No comments yet