Water hydrogen bonds tunnel in tandem
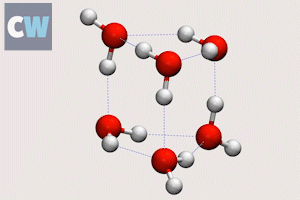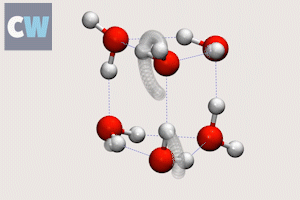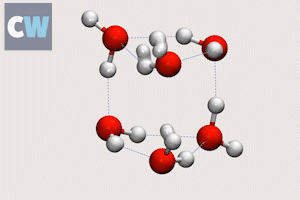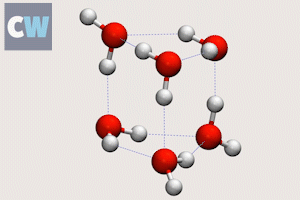
Cog-like interactions seen between molecules in ‘smallest droplet’ of water
By Simon Hadlington2016-03-29T00:00:00

Source: Jeremy Richardson
Cog-like interactions seen between molecules in ‘smallest droplet’ of water
Site powered by Webvision Cloud