What are the big problems for the next generation of chemists to work on? Mark Peplow takes up the gauntlet
‘We choose to go to the Moon in this decade and do the other things, not because they are easy, but because they are hard; because that goal will serve to organise and measure the best of our energies and skills.’ President Kennedy’s 1962 clarion call for the Apollo programme set out a breathtaking vision that mobilised the biggest scientific and technological effort in history. As grand challenges go, it’s hard to beat.
Audacious programmes for innovation didn’t start with Kennedy, though – Apollo was joining a long tradition. When the British government offered its longitude prize in 1714, for example, it stimulated the development of marine chronometers of unprecedented accuracy. And in 1900, German mathematician David Hilbert set the agenda in his field for a century when he posed his 23 ‘mathematical puzzles’. Bill Gates was inspired by Hilbert when he established a list of ‘Grand challenges in global health’ in 2003, and offered scientists almost half a billion dollars from the Bill and Melinda Gates Foundation to tackle them.
A grand challenge must go further than rhetoric about saving lives, saving the environment or saving money
The concept of the ‘grand challenge’ has become almost ubiquitous in science funders’ portfolios over the past two decades, covering a vast range of topics from robotic cars to oil spills. These big interdisciplinary projects are in vogue, not least because they can persuade governments to provide extra research funding, says Ben Martin, a science policy researcher at the University of Sussex in the UK.
What are chemistry’s grand challenges? These do not have to be the most philosophically profound questions – the chemical origins of life, for example – but they should lead to answers that can significantly improve the sum of human wellbeing. Grand challenges are more than a mere list of questions – they should have a realistic route to an ambitious but achievable goal. And a grand challenge must go further than rhetoric about saving lives, saving the environment or saving money – they should expect to command political support, attract sustained funding and capture the public’s imagination. The sheer complexity of these pervasive, global challenges might require hundreds or thousands of researchers to step outside of their normal mode of working and join forces across disciplines and continents.
Here, then, are five of chemistry’s grand challenges. Two overlap (coincidentally) with major initiatives being supported by the Engineering and Physical Sciences Research Council (EPSRC). A few chime with the broader societal challenges laid out in the Royal Society of Chemistry’s 2009 report Chemistry for tomorrow’s world. Some may be conquered in a decade; others seem like distant dreams. And the list is certainly not definitive – after all, how could such a brief summary encompass all that the chemical sciences can achieve? Instead, it offers a glimpse of some of the ways that chemistry can make the world a better place, one molecule at a time. As Kennedy said: ‘By defining our goal more clearly, by making it seem more manageable and less remote, we can help all peoples to see it, to draw hope from it, and to move irresistibly towards it.’
Build a synthesis machine

You’ve modelled the cell receptor, and assembled a shortlist of molecules that could be a perfect fit for the active site. You’ve drawn the structures on the computer, and topped up the reagent containers on the machine beside it. It’s late: you hit the flashing green button, and head home. In the morning, half a dozen small vials filled with clear liquids are waiting for you in the machine’s dispensing rack. Time to run those binding assays…
Our goal is the ability to make molecules on demand
Richard Whitby
If only it were that easy. Automated synthesis would revolutionise chemistry, but it will take some enormous breakthroughs to make it a reality. It requires a portfolio of reactions that work quickly and reliably in almost quantitative yields with no troublesome byproducts. These reactions should run sequentially, under continuous flow conditions, speeded by efficient catalysts. Perhaps most challenging of all, we would need to dramatically improve our understanding of chemical reactions so that a computer program can map out the most promising synthetic routes and then rank them on criteria such as atom economy and overall yield.
Many research groups are working on parts of this puzzle, but the EPSRC Dial-a-Molecule Grand Challenge, which began in 2010, brings them together into a unified, interdisciplinary effort. ‘Our goal is the ability to make molecules on demand,’ says Richard Whitby of the University of Southampton in the UK, who leads the challenge. ‘It’s one of the very few grand challenges that we haven’t copied from the United States,’ he adds. ‘Either we’re ahead of them or we’ve got it wrong.’
One of the key parts of the challenge is to spark a cultural shift in the way chemists approach their work, making it a more data-driven discipline. Rather than regarding the product as the primary output of a reaction, the real prize should be the data that can be gathered to explain exactly why it works – or, in most cases, doesn’t work, says Whitby. Electronic notebooks are one way to capture that slew of data, and another is mechanisation.
Flow chemistry, for example, offers close control over reaction conditions and in situ monitoring. In one afternoon, a student can work out the complete kinetic parameters of a reaction, something that might take days using conventional methods. That enables rapid optimisation of reaction conditions, and can potentially generate large numbers of analogues very quickly. ‘To do that manually is incredibly difficult because of the inherent variability of the human chemist,’ says Whitby. He notes that some disciplines within biology have changed radically in the past decade by embracing big data, and it has paid off in very rapid advances. In contrast, most of chemistry’s data remains hidden away in scrawled notes, or is never even collected. ‘We need to transform the way we do synthesis,’ he says.
It will take decades to develop a synthesis machine. But simply trying to develop these techniques, and changing the way chemists study their reactions, is an end in itself, says Whitby: ‘Any steps towards that have enormous benefit.’
Predict the future
One of the continuing scandals in the physical sciences is that it remains in general impossible to predict the structure of even the simplest crystalline solids from a knowledge of their chemical composition,’ wrote Nature editor John Maddox in 1988. Just over a decade later, the Cambridge Crystallographic Data Centre (CCDC) launched the first of its crystal structure prediction ‘blind tests’ to stimulate progress towards Maddox’s goal. The CCDC invited teams to predict the structures of a variety of organic molecules with known, but unpublished, crystal structures. Now preparing for its sixth contest next year, the blind test is the closest thing the field has to a grand challenge.
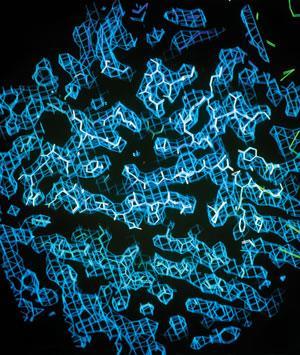
‘The solid form of a chemical has very different properties depending on its crystal structure,’ explains the CCDC’s David Bardwell, who coordinates the tests. ‘That can be quite a problem for the pharmaceutical industry.’ Different crystal polymorphs have different solubilities, for example, which can affect how a drug is absorbed by the body or complicate manufacturing processes. Predicting possible polymorphs can help to solve these problems before they occur. ‘In recent years we’ve made leaps and bounds, at least for smaller, more rigid molecules,’ says Bardwell. But larger, more flexible molecules – including many pharmaceuticals – still pose an enormous challenge. Sometimes hundreds of possible structures can have lattice energies very close to the crystal’s thermodynamic minimum.
Teams use a wide range of techniques, but they generally start by calculating the lowest-energy arrangement of atoms and bonds within a single molecule. Then they pack these computer-modelled molecules together in as many plausible ways as possible, accounting for all of the interactions between the molecules, and rank them based on their chances of matching real-world structures.
There are literally hundreds of thousands of ways that a protein can fold
Harold Scheraga
Throwing more computing power at the problem is not always the answer. ‘It’s a case of getting smarter,’ says Bardwell. Restricting the models to the most common of the 230 crystallographic space groups can narrow the options, as can surveying databases of known crystal structures to find the patterns typically adopted by the molecule’s functional groups. For now, structures generated this way can offer useful information to scientists who understand the limitations of the models, says Bardwell, but they cannot be relied upon unquestioningly. Accounting for solvent interactions offers one route to improving the predictions, he adds.
Crunching through all those possible crystal structures might be possible for small molecules, but it is completely impractical for proteins. ‘There are literally hundreds of thousands of ways that a protein can fold,’ says Harold Scheraga at Cornell University in New York state, US.
Accurately predicting protein structures has become a crucial goal in drug development. The structure will likely give clues to a protein’s function, and researchers can mould drug candidates to fit the binding site of a protein receptor, for example. Understanding protein folding can also reveal the mechanisms of diseases, since any change to the structure can drastically change its function – just think of the impact of the misfolded proteins implicated in Alzheimer’s disease.
The leading methods to predict protein folding rely on molecular dynamics, modelling how the protein’s atoms respond to the various forces around them as they settle into their lowest-energy arrangement. Coarse-grained models can simplify the task, by averaging the possible orientations of the amino acid side-chains, and of the peptide groups between each successive alpha carbon.
The main competition to test these methods is the Critical Assessment of Techniques for Protein Structure Prediction, held every two years since 1994. The structures in the latest competition were pretty accurate, says Scheraga, but they’re not yet reliable enough for rational drug design. ‘I think we have a pretty good idea of how to do it, it’s now a question of refining the output’.
Deal with carbon dioxide

Instruments at Mauna Loa in Hawaii have been recording the atmospheric concentration of carbon dioxide since 1958, when levels averaged about 316 parts per million (ppm). In May, levels there rose above 400ppm for the first time in several million years, and they are unlikely to stop rising any time soon. As the greenhouse gas accumulates in our atmosphere, it accelerates global warming, and at its current pace our planet’s climate will be radically altered by the end of the century.
To curb anthropogenic carbon dioxide emissions, we must reduce our dependency on fossil fuels by switching to renewable forms of energy (see below). We could also capture carbon dioxide from power plants’ flues – or wrest it directly from the atmosphere – and pump it deep into porous rock formations to stop it contributing to the greenhouse effect. But why not turn the troublesome gas into something useful?
CO2Chem, another of the EPSRC’s grand challenges in chemistry, focuses on carbon capture and utilisation. Its goals are to reduce emissions, and transform carbon dioxide into useful chemical feedstocks and fuels. ‘It’s about removing the chemical industry’s reliance on fossil fuels,’ says Peter Styring of the University of Sheffield, UK, who leads the effort.
The first challenge is to capture the gas. That is currently done using a solvent, such as monoethanolamine (MEA), which reacts with carbon dioxide at about 50°C to form a carbamate. Separating that and heating to about 120°C breaks down the carbamate, releasing carbon dioxide and regenerating MEA. That temperature swing makes the process energy-intensive and expensive. ‘If you retrofit that to a power facility, it consumes about 30% of the power output of the plant,’ says Styring.
Chemists developing alternatives to MEA are looking for low-cost materials that minimise those energy requirements, while selectively sucking up huge amounts of the gas. Some are using the tailored pores of metal–organic frameworks (MOFs) to grab carbon dioxide molecules, while others are incorporating it into minerals used in building materials. Styring says that ionic liquids are particularly promising, because they can be readily fine-tuned to minimise the temperature swing used to capture and release carbon dioxide. In principle, it should even be possible to incorporate catalysts that transform the waste gas into useful products such as hydrocarbons, which would then be released more easily. Styring’s team plans to set up a demonstration carbon capture unit next year using 1kg of ionic liquid.
Any country with this technology would become self-sufficient in fuel
Peter Styring
The main drawback of ionic liquids is their high cost. That could be mitigated somewhat if carbon dioxide became the one-carbon feedstock of choice in a wide variety of chemical processes. It is already used to make urea and salicylic acid, and in 2011 an Icelandic company called Carbon Recycling International started to use geothermal energy to convert the gas into methanol, producing around 2 million litres per year. Bayer MaterialScience has developed a process to turn carbon dioxide into polyols that are used in polyurethane production. BASF is working on doing the same for polycarbonates.
The key to broadening the use of carbon dioxide is to find efficient ways to break those strong carbon–oxygen double bonds. ‘You can find lots of examples of exotic catalysts that can transform CO2 at tiny scales, but they’re not suitable for scale up,’ says Styring. Researchers involved in CO2Chem are looking for cheap, robust catalysts, based on iron or aluminium, for example.
Styring has an infectious enthusiasm for his challenge, and is optimistic about its prospects. ‘There’s been an almost exponential growth in research in the past few years, much of it from China,’ says Styring. Besides the urgency of climate change, governments have a clear motivation to invest in this work, he adds: ‘Any country with this technology would become self-sufficient in fuel.’
Cut renewable energy costs
In just one hour, more solar energy falls on the Earth than we currently consume in a year. The Sun offers our brightest hope for a cheap and practically endless source of power – and chemistry has a vital role in harvesting it more effectively.

The US Department of Energy’s SunShot initiative is a grand challenge that aims to ‘make solar energy cost-competitive with other forms of electricity by the end of the decade’. Solar photovoltaic electricity is already drawing close to that goal: the average price of silicon photovoltaic modules has dropped 100-fold over the past four decades to about $0.74 (£0.50) per watt this year, according to consultants Bloomberg New Energy Finance. In recent years, cost reductions have largely come from economies of scale as the industry has grown, and prices should continue to fall. But chemists can help by improving silicon refining methods, and reducing the amounts of raw materials that go into the modules; and, as the industry expands, chemistry will have an important role limiting the environmental impact of producing the solar modules themselves. In the longer term, roll-to-roll processing could enable organic photovoltaics or dye-sensitised solar cells to be made at even lower costs than silicon modules.
Battery storage is still incredibly expensive. We have to drive down those costs
Elise Fox
SunShot also aims to improve solar thermal energy plants, which typically capture heat from sunlight to generate electricity or provide hot water. Elise Fox, a materials chemist at Savannah River National Laboratories in South Carolina, is working on this part of SunShot, and next year will chair the American Chemical Society’s Energy and Fuels division. She has been developing ionic liquids that contain nanoparticles such as alumina or carbon black, so that they can absorb and carry heat more efficiently. One of the biggest challenges is to ensure that these ionic nanofluids can stand up to years of hammering by the Sun’s rays, says Fox: ‘Over 2000 hours, some of these ionic liquids turn to tar.’
Many renewables produce an intermittent supply, so they must harvest energy at every opportunity and store excess power for later use. But most storage methods – such as generating hydrogen gas, or charging batteries – are not particularly efficient. Since the first commercial lithium-ion batteries reached the market in the 1990s, for example, improvements have been fairly incremental, says Fox. ‘Battery storage is still incredibly expensive,’ she says. ‘We have to drive down those costs.’
Lithium–air batteries are a particularly promising alternative, according to Fox. They have a very high energy density and are much lighter than conventional lithium-ion batteries because they do not have to carry their own oxidiser. But a build-up of insoluble lithium peroxides inside the battery can prevent metal ions from migrating to the anode, which reduces the battery’s electrical capacity with each recharge. Fox is developing manganese oxide catalysts that can prevent that peroxide build-up. ‘Improving the rechargeability of lithium air batteries would be a huge bonus,’ she says.
Make sense of genetics
‘Chemical biology isn’t something new,’ says Shankar Balasubramanian of the University of Cambridge, UK. ‘Chemists have been contributing to biology for more than a century.’

Balasubramanian should know. In the late 1990s, he and his colleagues began to develop a new method of sequencing DNA – working out the precise order of the cytosine (C), guanine (G), adenine (A) and thymine (T) nucleobases that encode information in our genomes. The technique uses four different nucleotides tagged with fluorescent molecules to build up a complementary strand of DNA, one base at a time. After each addition, a laser cleaves the fluorescent tag, which emits light, revealing which base has just been incorporated. The team founded a company, Solexa, to commercialise the technology – it quickly became one of the leading sequencing methods, and has helped to deliver an avalanche of data that has revolutionised genetics. Now, Balasubramanian is exploring a new frontier: epigenetics.
Epigenetics seeks to understand heritable genetic changes that do not involve altering the underlying DNA code. Take histone proteins, for example: these biological bobbins wind up the DNA double helix to pack it into chromatin, the building blocks of chromosomes. Chemical modifications to histones – with methyl or acetyl groups, for example – change the density of chromatin, which determines whether or not the genes held inside are expressed. Similar modifications affect the DNA molecule itself. About 2–5% of the cytosine bases within a genome have been altered by DNA methyltransferase, creating a new base called 5-methylcytosine that tends to reduce the expression of the genes it appears in.
Understanding how these epigenetic ‘marks’ change gene expression could explain how a dazzling spectrum of different tissue types spring from the same genome; and how external factors, such as nutrients or toxic chemicals, can leave their imprints on our bodies. Although these marks are largely stripped away as embryos form, some can persist – including those that confer longevity or increase disease risks, according to animal studies.
We know very little about the chemistry of how the genome changes
Shankar Balasubramanian
In the past few years, researchers have also found that 5-methylcytosine can be oxidized to 5-hydroxymethylcytosine, then 5-formylcytosine, and finally 5-carboxylcytosine. On top of the canonical A, G, C and T, ‘that’s four more bases’, says Balasubramanian. Scientists are only just beginning to understand the impact of the three oxidised forms, but they appear to be linked with changes in enzyme activity, and some are highly correlated with certain cancers. ‘It does raise the question of what else we haven’t seen,’ says Balasubramanian.
Last year, he and his colleagues unveiled a sequencing technique that could map 5-methylcytosine and 5-hydroxymethylcytosine at single-base resolution, offering a powerful new way to probe epigenetics. Meanwhile, the Human Epigenome Project is currently trying to map the locations of all possible epigenetic changes across the whole genome. The results could pave the way for disease therapies that target epigenetic marks, or reveal how certain environmental pollutants raise our risk of disease. ‘We know very little about the chemistry of how the genome changes,’ says Balasubramanian. ‘Chemistry will make a very important contribution in this area.’
Mark Peplow is a science journalist based in Cambridge, UK












No comments yet