Our sense of touch and balance is deeply ingrained in our experiences, but what are the chemical processes that make it work? Rachel Brazil investigates
Try closing your eyes and touching the tip of your nose with your finger. Most of us can do this easily: we have the natural ability to sense our own muscles stretch and this gives us a sense of balance and allows us to control our movement. But what gives us this sense of what our bodies are doing, known as proprioception? Until recently it wasn’t clear, but in 2010 the protein family responsible for this was discovered. It turns out these proteins also allow us to sense touch and a whole lot more.
The family was identified by neuroscientist Ardem Patapoutian at The Scripps Research Institute in California, US, and he named the two related ion channel proteins Piezo1 and Piezo2 – from the Greek word for pressure. In the last seven years it has become apparent that these Piezo proteins are a fundamental part of biological control systems.

‘We are really only looking at the tip of the iceberg regarding their physiological function,’ says Jörg Grandl, neurobiologist at Duke University in North Carolina, US. ‘It’s hard to find any cell in the body that does not have a few Piezo ion channels.’ While their importance is clear, the exact structure and functionality of these impressive mechanosensor proteins is an open question.
Ion channels are pore-containing proteins, found in cell’s lipid membranes. They open and close to control the flow of ions into and out of the cell, triggering cell signalling. Many are voltage-gated – activated by changes in the electrical membrane potential near the channel – but Piezo ion channels open in response to mechanical stimuli, allowing sodium, potassium and calcium ions into the cell. Of the two human forms discovered, Patapoutian confirmed Piezo2 as the channel responsible for our sense of touch in 2014.1 His team engineered mice without the Piezo2 protein in their sensory neurons and skin cells and found they were unable to feel in several touch stimuli tests.
Patapoutian confirmed the link between Piezo2 and proprioception also using engineered mice, but this time silencing Piezo2 in the neurons that sense the stretch of muscles.2 The mice were unable to walk normally. Mutations of the protein have also been linked to rare genetic conditions that leave people without proprioception.
Piezo proteins are not the first ion channels discovered that respond to mechanical force. ‘That distinction belongs to a family called TREK – a potassium-selective mechanically sensitive channel,’ explains Philip Gottlieb from the State University of New York in the US. He has been studying ion channels for almost 20 years with ‘father of the field’ Frederick Sacks, who in the mid-1980s discovered ion channels can convert mechanical stress into electrical or biochemical signals. TREK, one of these mechanosensitive ion channels, is found in brain and heart nerve cells and seems to have an important role in protection against epilepsy.
Mechanically triggered ion channels in bacteria have also been extensively studied since their discovery in the late 1980s. The channel MscL (the large conductance mechanosensitive channel) acts as an emergency valve and stops bacteria swelling up and bursting on sudden exposure to water. When open, it allows through molecules with molecular weights of up to 1000Da to regulate the bacteria’s osmotic gradient.
‘Most proteins sense mechanical forces on some level,’ explains Patapoutian, but ‘the distinction we make is between proteins that are modulated versus activated by mechanical forces’. While pressure can affect other ion channels, Piezo1 and 2 are primarily designed to respond to mechanical, not chemical stimuli. Most cells communicate through chemicals, but sensory neurons need to be responsive to other signals – such as temperature or pressure – and be able to translate these physical stimuli into electrical impulses in milliseconds. Before discovering Piezo proteins Patapoutian’s lab had already discovered the first cold-activated ion channel (which also acts as a menthol receptor, explaining the cold sensation the compound creates).
‘But at some point we realised that the elephant in the room was mechanosensation’ says Patapoutian. There was little understanding of our sense of touch. ‘We knew that there were ion channels that are mechanically activated, we knew where they would be, but their molecular identity was not known.’
Piezo proteins look nothing like anything we know, which makes studying them really hard
Jörg Grandl
Patapoutian set about changing this by identifying a mouse or rat cell line that showed evidence of mechanically activated currents. He did this using the well-established patch-clamp electrophysiology technique, which uses a glass micropipette electrode to suck up a small part of the cell membrane. The voltage is read against a reference electrode in the surrounding cell bath. When mechanical pressure is applied to the cell surface, any changes to the ion channels state can be probed.
Using bioinformatic data, the most likely proteins were identified and screened using small interfering RNA (siRNA) knockdown. Small 20–25 base pair RNA molecules are introduced that block the transcription of a specific protein, and in its absence it’s easy to see if the mechanically activated currents disappear. They found FAM38A – which was renamed Piezo1.3 The final most important test transected Piezo1 into other cell lines, which then gained mechanosensitivity. ‘This told us that [the Piezo ion channel] is not just necessary but also sufficient to transmit mechanosensitivity.’ says Patapoutian.
The next task was to see if Piezo1 has brothers or sisters. Comparing mammalian genomes, they found one other protein that was almost 50% identical, which they named Piezo2. And genomic analysis also predicts Piezo channels in many other organisms, even plants and slime moulds.
Unique proteins
‘Piezo proteins are unlike any other ion channels’ says Grandl. ‘They are completely different in their amino acid sequence, their protein sequences and presumably their structure. They look nothing like anything we know, which makes studying them really hard.’
Firstly they are unique in their size: both Piezo1 and 2 are massive proteins (2521 and 2752 amino acids respectively), the largest ion channels discovered to date, at around 200Å in diameter. Modelling predicts over 14 transmembrane domains that weave their way through the cell membrane – also more than most ion channel proteins. These features are likely linked to the mechanism by which it senses stretch and mechanical force, Patapoutian says, but exactly how it works is still unknown.
In 2015 Bailong Xiao of Tsinghua University in Beijing published electron microscopy imaging of mouse Piezo1 at cryogenic temperatures4 providing some further clues to the enigmatic structure. The channel forms a trimeric complex, with the central pore surrounded by a unique three-bladed propeller-shaped architecture. It appears that the ions pass through the interface between the three sub-units with the central pore topped by a cap which has an unusual folded structure, not found elsewhere in nature.5
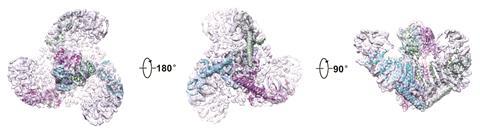
So far, the structural information gathered is only giving clues to how Piezo channels open and close. There is a complex apparatus fashioned from the large protein molecule but its not yet clear which parts act as the sensing trigger. One question has been whether the protein works independently within the cell membrane or if it is anchored to the cell’s own internal scaffolding. Gottlieb and colleagues have answered this by creating ‘blebs’ – outgrowths in cell membranes disconnected from the cell’s internal cyctoskeleton proteins where Piezo1 channels were still active.6
So the proteins sense pressure directly from the membrane, and this might also explain their large size and membrane coverage, suggests Grandl. ‘That might help them be very sensitive to what’s going on in the membrane.’ One suggestion is that pressure on the membrane dents its thickness, which causes a ‘hydrophobic mismatch’ with the hydrophobic regions of Piezo that normally sit within the membrane. The exposure of these hydrophobic parts of the protein could lead to a change in protein conformation that opens the ion channel.
Part of the challenge is developing the right method to probe the protein on a submolecular level. ‘We are really only beginning to develop good tools to apply mechanical forces that are really small – we are talking about forces in the order of piconewtons – that’s a billionth of a newton,’ says Grandl. He is using magnetism to try and work out which parts of the protein’s many domains are pressure sensitive.
Grandl has engineered Piezo proteins to contain an additional 13 amino acid sequence within the region of the protein being tested. This sequence could bind to 75nm magnetic nanoparticles coated with the protein streptavidin. By applying a focused magnetic field he could then apply a force specifically on one protein domain whilst measuring channel activity.7 ‘Using this approach we were able to scan through the entire protein and identify two domains that seem to have unusually high mechanical sensitivity,’ says Grandl. One of these was near the pore itself, but the other was located at the opposite end of the protein, which Grandl suggests could mean the ion channel also operates with some sort of distant lever mechanism.
Beyond touch
The race to solve the puzzle of the ion channel’s structure and mechanism continues, as does the mission to understand the ever growing biological roles that Piezo proteins seem to play. Piezo2 was first identified as the ion channel mediating our sense of touch and proprieception, but it turns up in other sensory cells and seems to be crucial for all types of mechanical feedback. For example Piezo2 seems to be involved in sensing lung stretch within lung tissue, to control breathing patterns. It is also involved in the mechanisms that control gut motion, through seratonin release. It’s even been found in knee cartilage – it seems wherever there are moving parts you will find Piezo proteins.
Piezo1 is primarily found in non-sensory cells and organs exposed to fluid pressure. It is found extensively in the cardio-vascular system, including red blood cells. ‘Red blood cells experience a massive amount of mechanical force as they navigate through blood vessels and apparently Piezo plays an important role in sensing this and regulating cell volume, depending on where they are in the cardio-vascular system,’ Patapoutian explains. He says this may explain how blood cells are able to squeeze into very narrow capillaries. Piezo is also crucial for embryonic development, with Piezo1 playing a role in vascular growth. Mice engineered without it do not develop a normal vascular system and will not survive.
Kinetics and disease
Piezo proteins seem to do so much, but why are there two different versions of the protein in mammals? From modelling data they both have similar structures and are both pressure-sensing and allow multiple cation types through their pore. Grandl suggests that Piezo1 may be sensitive to a larger number and types of mechanical forces, whereas Piezo2 is more narrowly tuned to specifically detect mechanical touch. ‘One of the few differences is their inactivation kinetics’ says Patapoutian. Once stimulated some ion channels will automatically become inactive and effectively close down ion flow, regardless of continued stimulation. ‘Piezo2 inactivates a little bit faster than Piezo1, again you can look at this and say these are not big differences, but activation kinetics is actually very important. That clue comes from mutations found in humans,’ he adds.
Since its discovery, a number of rare genetic diseases have been linked to mutations in Piezo proteins and some of these are caused by changes in ion channel kinetics. If the inactivation mechanism is slowed down, this causes larger ion flows into the cell than required, known as a gain-of-function. This is what happens in the genetic disorder hereditary xerocytosis,8 a form of anaemia linked to mutations in red blood cell Piezo1 channels.
Gottlieb looked at Piezo1 activity in cells with the mutation. ‘There were significant differences in what we call the open to the inactivated state – it turns out it was shutting down slowly and so that led to more ions coming through than there should have been.’ The excess calcium influx into red blood cells causes downstream activation of osmotically driven dehydration, which damages the cells and leads to the disease.
Pain
So what about pain? Does the touch-sensitivity afforded by Piezo2 channels also extend to the sensations that cause pain? Obviously a better understanding of how pain occurs is high on the list of priorities for researchers looking at ion channels. A link might not have been a surprise – but the relationship doesn’t seem to be that simple. ‘When you delete the Piezo2 protein from neurons, mice are completely insensitive to light touch, but they seem to respond to normal high intensity touch which is thought to elicit pain,’ explains Grandl. ‘Its very clear that the principle mechanosensor invovled in pain is not Piezo2’ adds neurobiologist John Wood, from University College London in the UK. Wood worked with Patapoutian to show this.
When you hit your thumb with a hammer, we don’t know what that channel is that makes you go ouch
John Wood
But Patapoutian isn’t totally discounting any relationship: ‘The jury is a little bit out on this,’ he says. It’s an area his group are actively working on. Wood says there may be a link to neuropathic pain – pain due to tissue injury, where nerve fibres may be damaged or dysfunctional.
Wood, whose work explores the physiology of pain perception, says pain is tricky and not just simply the response of a cell sensor. ‘Pain is a perception or sensation in your brain that we don’t understand.’ It does seem likely that there is another mechanically activated ion channel with a higher threshold that mediates pain, but the molecular identity is not known yet. ‘This is the exciting thing,’ adds Wood. ‘When you hit your thumb with a hammer, we don’t know what that channel is that makes you go ouch – so that’s still up for grabs and is a very interesting prospect.’
Whilst the tantalising prospect of finding a new pain receptor and molecules that could inhibit it is in the future, researchers have been looking for molecules that might be able to inhibit Piezo ion channels to treat the diseases in which Piezo proteins play a role. Even before the proteins were identified, Frederick Sachs and his team had identified a small peptide from the venom of the Chilean rose tarantula that blocks mechanosensitive ion channels, including Piezo1. The versatile molecule is in preclinical development as a therapy for the muscle-wasting disease Duchenne muscular dystrophy (DMD). It has been linked to leaky mechanosensitive ion channels, causing calcium influx into cells which leads to muscle degeneration. By inhibiting those ion channels, a balance can be restored.
But given its universal nature, it is debatable how useful Piezo blocking drugs will be. ‘They are playing so many different important roles that a global activator or inhibitor might have many different side effects,’ says Patapoutian.
Given that Piezo proteins were only discovered seven years ago, there is already a massive body of work showing its importance in much more than our sense of touch. Their use across many organs illustrates biology’s ingenuity and economy. Though clues are starting to pile up, exactly how Piezo proteins work on a molecular level is still a puzzle. ‘It will be very interesting and very exciting in the next 10 years to figure all of that out,’ says Grandl.
Rachel Brazil is a science writer based in London, UK
References
1 S S Ranade et al, Nature, 2014, 516, 121 (DOI: 10.1038/nature13980)
2 S-H Woo et al, Nat. Neurosci., 2015, 18, 1756 (DOI: 10.1038/nn.4162)
3 B Coste et al, Science, 2010, 330, 55 (DOI: 10.1126/science.1193270)
4 J Ge et al, Nature, 2015, 527, 64 (DOI: 10.1038/nature15247)
5 A Kamajaya et al, Structure, 2014, 22, 1520 (DOI: 10.1016/j.str.2014.08.009)
6 C D Cox et al, Nat. Commun. 2016, 7, 10366 (DOI: 10.1038/ncomms10366)
7 J Wu, R Goyal and J Grandl, Nat. Commun, 2016, 7, 12939 (DOI: 10.1038/ncomms12939)
8 R Zarychanski et al, Blood, 2012, 120, 1908 (DOI: 10.1182/blood-2012-04-422253)












No comments yet