Making drugs to treat animals is potentially lucrative – but also difficult, as Clare Sansom discovers

Chocolates, grapes, raisins and onions are some of our favourite foods: eating them is unlikely to make many of us very ill. But leave a piece of your favourite chocolate cake within reach of your dog, and you may find your beloved pet suffering from acute renal failure. The chocolates that we love contain theobromine, which dogs metabolise very slowly so it builds up into dangerously high concentrations. Chocolates sold for dogs have been specifically formulated without this compound. Chemicals in raw onions set up a reaction in both dogs and cats that prevents their red blood cells from carrying oxygen round the body.
These examples illustrate the significant biochemical and metabolic differences between humans, dogs and cats – and, indeed, between all mammalian species – that make the design of medicines for use in animals a challenging endeavour. And veterinary drug development is further complicated for animals that are to enter the human food chain. The development process for livestock drugs involves long-term toxicity tests in rodents – often encompassing the whole of the natural rodent lifespan – as well as in other mammals, so that farmers and consumers can be wholly confident that the meat, milk or eggs will contain no potentially harmful drugs or metabolites. Furthermore, as Jonathan Elliott, professor of clinical pharmacology at the Royal Veterinary College in London, UK, explains, ‘all drugs to be prescribed for livestock are labelled with a minimum withdrawal period after treatment ends before an animal may be sent for slaughter, or milk may be sold’.
Concerns about the environmental consequences of field run-off must also be taken into account in developing drugs that are to be administered to large numbers of farm animals, such as flukicides for controlling liver fluke in sheep. And human safety is also a concern in developing medicines for pets (or ‘companion animals’ as professionals describe them). Drugs must often be administered by untrained pet owners in their homes where they may be accidentally consumed by young children. Further difficulties arise from the need to formulate compounds that are palatable to animals, and to devise delivery mechanisms that will work even for animals that may be highly stressed. Each species needs a range of medicinal products that is different in terms of both molecules and formulations.
For all these reasons, veterinary drug development offers challenges that are at least as difficult as those in the development of human drugs. Many of the large pharmaceutical companies nevertheless have, or have had, some involvement in animal health. The medicines they produce are as essential as human drugs: both in supporting vets and livestock farmers to provide a safe, wholesome supply of meat and dairy food, and in supporting vets and pet owners to help companion animals live longer and healthier lives.
Development difficulties

Occasionally, a novel molecule from a class commonly used in human medicine will prove effective in treating animal disease. Zoetis, the former animal health business of giant pharmaceutical company Pfizer, licensed its first canine oncology drug in 2009: Palladia (toceranib) for the treatment of mast cell tumours, which are particularly common in older dogs. This is a receptor tyrosine kinase inhibitor, which puts it in the same drug class as Glivec (imatinib) and several other widely used human oncology drugs, but it is not itself used for any human cancer.
‘Mast cell tumours can be debilitating in dogs, and Palladia represents a therapeutic advance in comparison to the previous standard of care which was based on human cancer therapeutic cocktails,’ says Michael Stegemann, senior director for global development and operations at Zoetis. Palladia has greatly improved the treatment options available, but its success, like that of so many drugs for human cancer, has to be measured in extended lifespan and improved quality of life rather than in terms of complete cures.
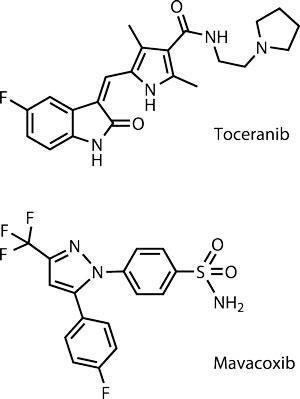
Another drug class that has successfully crossed from human to animal use is the coxibs: anti-inflammatory drugs that work by selectively inhibiting the enzyme COX-2. Zoetis’ Trocoxil (mavacoxib) is available only for dogs. ‘Trocoxil’s unique pharmacokinetic behaviour allows a once-a-month oral treatment after an initial 14-day treatment interval, and thus addresses both compliance issues and the chronic nature of canine osteoarthritis,’ says Stegemann.
It is unlikely, however, that antibodies and other protein therapeutics equivalent to those that have revolutionised the treatment of some human diseases will be widely used in animal medicine in the near future. The process of ‘caninising’ or ‘felinising’ a mouse monoclonal antibody is the same as that of humanising it, but it is still a painstaking and expensive one. Similar concerns arise with erythropoietin, a protein that is used to treat kidney disease which is particularly common in elderly cats. ‘Cats and dogs with kidney disease are treated with recombinant human erythropoietin, which is reasonably effective but can cause antibody development; a canine or feline equivalent would be preferable, but it will not be cost-effective to develop one as the market is not large enough,’ says Elliott.
The development and registration processes for all types of veterinary drugs are similar to those for human ones. Drugs are first tested in non-target species, with the decision whether to take a compound into the target species made earlier in the process than the equivalent for a human drug. The first ‘clinical trials’ of a veterinary pharmaceutical are dose determination studies using a variety of dose regimens with a small number of experimental animals of the same species as the target species, held in controlled conditions that simulate the naturally occurring disease. Once the optimum dose has been determined, a dose confirmation study is performed in a larger number of experimental animals, also in controlled conditions. Final confidence in a product’s efficacy and safety comes from field safety and efficacy trials using animals in natural conditions with the disease that the product is designed to treat. All products will continue to be monitored for efficacy and adverse events after approval.
Veterinary drugs are approved by specialist branches of regulatory agencies such as the Center for Veterinary Medicines (CVM) at the Food and Drug Administration in the US. Each drug will only receive approval for use in the species and for the indication in which safety and effectiveness has been confirmed. ‘The pharmacology of each species cannot be assumed to be identical and it is inappropriate to treat a cat as though it were simply a small dog,’ explains Marilyn Martinez of the Office of New Animal Drug Evaluation at the CVM. ‘In addition to metabolic and biochemical differences, there are substantial differences in their respective gastrointestinal systems and in their taste preferences. For this reason, it is important that each drug be tested in the respective animal species and that the formulation is developed with individual species’ preferences in mind. In this regard, most cat owners will say that product acceptability can be particularly challenging in the cat.’
Bees’ disease

Bees are the only invertebrates to feature in veterinary medicine, and the idea of bee medicine is still a recent one. Common bee pests include the varroa mite, which can be treated with pyrethroid and organophosphate insecticides, and American and European foulbrood (AFB and EFB). In the UK, honeybee colonies diagnosed with a mild form of EFB are treated with antibiotics such as oxytetracycline. There is no legal treatment for more severe cases, however, and severely affected colonies are destroyed by fire. In the US, however, antibiotics are legally available for treating both AFB and severe EFB. And bee medication may become even more important as the prevalence of colony collapse disorder grows.
This is a strange phenomenon in which the worker bees from a beehive or colony disappear abruptly, and it was first observed in North America in 2006. While some attention – and most media coverage – has focused on pesticides several pathogens including bacteria, viruses and the varroa mite have also been implicated. If a pathogen does turn out to be responsible it should be treatable, as antimicrobials have been developed for other bee diseases. The resulting honey, however, may be subject to the same kinds of control as milk from cows that had been treated with antibiotics.
A question of breeding
In May 2013, the CVM website listed 634 separate products licensed for use in dogs but only 313 in cats. This statistic might be thought surprising, because the cat is the more popular US pet: more households have cats, and there are an estimated 82 million in the country, compared with 72 million dogs. There is also more active research into canine medicines. Martinez is unsure of the reasons for this disparity. ‘I know that veterinary practitioners have expressed the need for more feline-specific drug products. Nevertheless, the number of drug applications for dogs continue to markedly exceed those submitted for cats.’
One complication in developing medicines for dogs is their marked inter-breed physiological heterogeneity. This is readily recognized in the differences in body size, which ranges from less than 3kg for an adult chihuahua to over 100kg for a Saint Bernard. Inter-breed differences also exist in disease propensities, with certain breeds being at higher risk than others for diseases including arthritis, cardiovascular diseases, ophthalmic problems and some cancers. There are also differences in drug metabolism, and one of the most frequently recognised of these occurs with the antiparasitic drug ivermectin. In some dogs, a defect in a transporter (P-glycoprotein) embedded in the blood–brain barrier allows this compound to build up in the brain, potentially causing severe neurological toxicity. This sensitivity has been traditionally viewed as a problem with collies but it is now recognised that it can extend to other breeds.
Veterinary pharmacologists therefore need to characterise differences in drug dose–exposure–response relationships both between species and across breeds, and particularly in drugs that need to be taken for long periods. It is, however, difficult to determine which breed variations are statistically significant due to limitations in the size of the study population. Importantly, basic research efforts into genetic polymorphisms in drug transporters or drug metabolising enzymes (pharmacogenomics) are not well funded in veterinary species, leaving large gaps in our basic understanding.
Martinez is currently working with software company Simcyp, a subsidiary of the UK-based Certara, to develop software that is specifically designed to simulate canine populations. Using available information and ‘what if’ scenarios (sensitivity analysis), this software will simulate drug pharmacokinetics across several canine breeds. ‘There are many gaps in our knowledge of variability in drug exposure across the canine population,’ says Martinez. ‘This software will be an invaluable tool to the veterinary community, helping to frame areas needing additional basic research, helping us develop the pre- and post-marketing information that must be submitted to the FDA, and helping our counterparts in human medicine understand variability and so improve their own drug development processes.’
The future
During recent years, complete genome sequences for all important veterinary species, livestock as well as pets, have been made publicly available. It is, however, fair to say that genomics is currently finding far fewer applications in veterinary than in human medicine, and then only at the earliest stages of drug development. For example, researchers are using transposon mutagenesis of bacterial pathogens such as Actinobacillus, which can cause pneumonia in pigs, to identify the most protective antigens to include in vaccines, and exploring the genetics and precise mechanism of the defect that leads to resistance to the antiparasite drug ivermectin in dogs. ‘We are already seeing how genomics can be a useful tool in drug design and screening,’ says Kevin Greenlees, senior advisor for science and policy at CVM’s Office of New Animal Drug Evaluation. ‘I am sure it will come to have applications in every stage of the development pathway.’

The global market for drugs for livestock is very different from that for pet drugs. The most important difference is that the range of products available is much more limited; for instance, although the FDA lists 688 products available for cattle in the US, a great majority of these are either anti-infective or anti-inflammatory drugs. Medications for diseases of ageing such as cancer and arthritis are not developed for livestock, as these species do not live long enough to contract such diseases.
In contrast to pets, when drugs are prescribed for cattle, pigs, sheep or poultry they may be given to the whole flock or herd at once, including in the feed. This method is much easier for a farmer or vet to administer, but, like any method of administration, it has its disadvantages. Specifically, it is unclear how responsible the use of antibiotics in livestock is for the current rise in antibiotic resistance. Proponents of general antibiotic use in livestock note that those that are most widely prescribed are from classes that are rarely if ever used in human medicine, such as the polyether ionophore monensin that is used to treat parasitic infections of cattle, and resistance to these does not cross over to humans. The debate on whether current practices allow livestock to be fed too many antibiotics is still ongoing and local regulations governing it vary substantially between countries and regions. Livestock in the European Union may only be given feed containing antibiotics if it has been prescribed by a vet; US regulators are currently developing a similar strategy for regulating medically important antibiotics in feed or water for livestock.
The fact that the principles of drug discovery are being applied to diseases of honeybees (see box) suggests that it will eventually be possible to overcome many of the challenges inherent in multi-species pharmacology. Veterinary pharmaceutical companies and their public sector counterparts are continuing to innovate, and drug development for animals, most particularly for dogs and cats, will continue to advance in step with its human counterpart. One slight brake on this innovation may simply be the amount that farmers, pet owners and insurers are able to afford to pay for novel drugs.
Clare Sansom is a science writer based in London, UK












No comments yet