Chemists are helping palaeontologists discover the rich palette of pigments in fossils, as Emma Stoye discovers
Fossils have given us unique snapshots from the long and complicated history of life. But we still know frustratingly little about the weird and wonderful species that roamed the earth millions of years ago. While fossils allow us to estimate when and where they lived, there is a lot they have not revealed. How did these extinct creatures behave? And what did they really look like?
Piecing together the patchy evidence has always been a multidisciplinary effort. Chemists’ best known contributions to palaeontology are probably radiocarbon dating and stable isotope analysis. But they now face a bigger challenge that has traditionally fallen into the hands of artists: adding colour to the ancient world.
‘The chemistry of life is like poetry,’ says Phillip Manning, a palaeontologist at the University of Manchester in the UK. ‘By the time it’s been buried for a few million years that poetry is still there, it’s just hard to read.’ To continue his analogy, all that remains of the biochemical poetry that determines colour are a few fragmented sentences and jumbled letters. Trying to rebuild these into something that makes sense is by no means easy.
For decades, the reconstructed portraits of dinosaurs and other lost creatures we still enjoy in museums and movies were based on little more than educated guesswork, often with a generous helping of imagination. After all, fossils themselves are not particularly colourful. ‘Most tend to be very monochrome or sepia coloured,’ says Mike Benton from the University of Bristol in the UK. ‘Although there may be hints of patterning – such as stripes and spots – and people used to speculate this may be representative of the original pattern, though they were never sure.’
Pigment of imagination
Things are starting to change, largely thanks to a single discovery: melanin, one of life’s most abundant colour-determining pigments, survives fossilisation. Melanin is responsible for the colour of hair, skin, feathers and fur, among other things. It is stored in microscopic vesicles called melanosomes in the fur and feathers of modern animals, and can be spotted easily using a scanning electron microscope (SEM). The fact that these structures can persist in fossils was discovered in 2008 by Jakob Vinther, then a PhD student at Yale University in the US.
Vinther, who is now based at the University of Bristol, was working with squid fossils at the time. He noticed that under the SEM their ink sacs were pretty much identical to those from modern squids. ‘You can clearly see these little melanin granules,’ he says, ‘which got me thinking: if melanin is preserved in squids, it should be preserved elsewhere in the fossil record.’
He began to search for melanosomes in exceptionally well-preserved soft tissue fossils. Feathers were an ideal starting point, and SEM imaging of fossil birds revealed sheets of preserved melanosomes populating the dark regions – again, identical to their modern counterparts. ‘People had of course seen these structures before, but they misidentified them as bacteria,’ says Vinther. ‘I guess I was coming at it from a different angle, because I was thinking that melanin should be preserved in these fossils.’
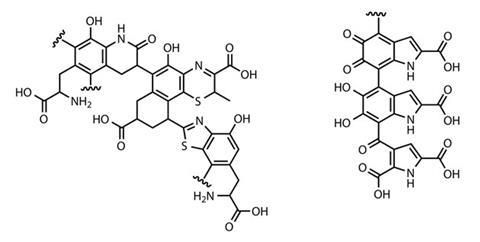
The presence of melanosomes not only indicates patterns of light and dark, but can actually give important clues to the organism’s original colour, says Vinther. ‘Different melanosomes have very distinct morphologies, so we can say something about colour simply by looking at the shape of these structures,’ he explains. Oblong, sausage-shaped melanosomes, for example, typically contain eumelanin – a tyrosine-derived nitrogenous polymer – which appears dark brown or black. More spherical melanosomes contain sulfur-rich pheomelanin – the melanin found in our lips and hair that gives a pink, red or russet colouration. ‘We developed a dataset for modern birds of different colours, which gave us a statistical way to measure what kind of colour a bird or feathered dinosaur had from melanosome shape,’ says Vinther.

As a result, feathered dinosaurs have been a popular candidate for this type of analysis. Benton’s group were working on early Cretaceous fossils from north-east China around the time Vinther’s first studies were published. His team identified eumelanosomes and pheomelanosomes in a number of specimens, including the feathered dinosaur Sinosauropteryx, which they deduced to have had ginger and white stripes.1 Vinther himself has been able to colour in the plumage of one of the most ancient known feathered dinosaurs, Anchiornis huxleyi, giving it a black and white body with a red Mohican.2
Vinther was also part of the group that uncovered direct chemical evidence of preserved melanin for the first time using a suite of analytical techniques to compare ground-up fossil squid ink to eumelanin-rich ink from modern cephalopods.3 Gas chromatography and mass spectrometry (GC-MS) showed both inks generated similar degradation products during a standard hydrogen peroxide assay. Fourier transform infrared spectroscopy revealed that the fossil ink contained a high proportion of carbon–oxygen double bonds, which is characteristic of eumelanin. The group also detected a free radical signature unique to eumelanin in the fossil ink using electron paramagnetic resonance spectroscopy. ‘We actually found that there’s about 10–15% pristine melanin inside these ink sacs which are 160 million years old,’ says Vinther. ‘It has a really strong propensity to survive.’
Crucial copper
Traditional chemical analysis requires samples to be chipped off from fossils, so it is impossible to investigate valuable specimens in this way. And methods involving the visual identification of melanosomes require extrapolation, as only tiny areas can be surveyed at a time.
‘You’d have to spend about 300 years mapping the whole [fossil] surface!’ says Manning, whose multidisciplinary team at Manchester has taken a different approach to searching for melanin in fossil feathers.

Their method analyses fossil chemistry without the need to take samples or damage the specimen. The team use extremely high intensity x-rays from the Stanford Synchrotron Radiation Lightsource in the US to scan the surface of a fossil and generate a map of elemental traces. ‘We had to then go back and scan tissues from living birds and look at where the concentrated trace metals were and what they meant,’ says Manning. The team identified organically bound copper as a potential biomarker for eumelanin. In modern feathers, says Manning, eumelanin chelates copper during its formation. The presence of organically bound copper in fossil feathers, therefore, correlates to darker colouration.
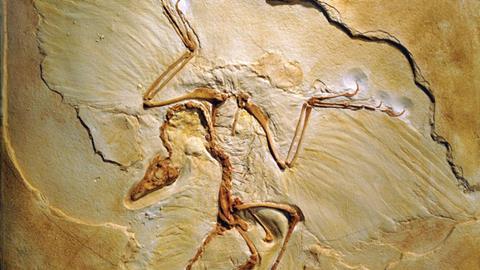
The team has used the technique to map the patterns of pigment in several feathered fossils, including the 150 million year old dinosaur Archaeopteryx,4 which was hailed as the missing link between dinosaurs and birds when it was discovered in 1870. The reconstruction they produced even added to the long-running debate over whether it was able to fly. ‘Pigmentation is concentrated on the edges and tips of the feathers, which is where you get maximum stress during flight,’ he says. ‘Melanisation strengthens the keratin in feathers, so this looks like an adaptation.’
But synchrotron scanning is not without its flaws. So far, only dark eumelanin can be mapped in this way, as there are no biomarkers for the other kinds. Some critics even doubt that copper is a reliable biomarker for eumelanin. Ryan Carney at Yale University in the US has also worked on Archeopteryx feathers, and his work, based on melanosome distribution, indicated its plumage was completely black.5 ‘In this case the chemistry contradicts the morphological evidence – they’re calling half of the feather white, but we found direct evidence of black pigment structures in that area,’ says Carney. He points out that copper could indicate the presence of some other organic compound, or could have been incorporated into the specimen from elsewhere.
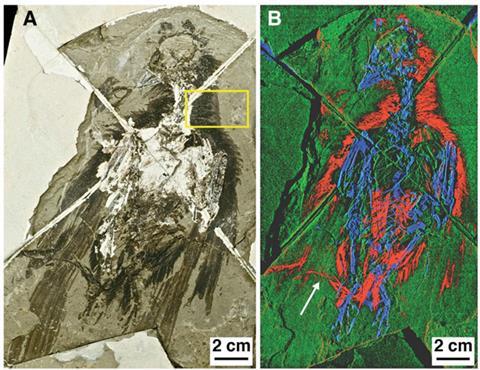
Manning argues this is not the case. ‘The oxidation state of copper that has been precipitated from the environment is totally different to what we see in organically bound copper,’ he says. ‘Only within the fossil do you get organically bound copper, and it matches precisely the organically bound copper we see in feathers with melanin.’
His group are now working on finding suitable biomarkers for other pigments so future reconstructions can go beyond monochrome. Manning also says that this type of analysis could be used to investigate other biomolecules. ‘We have scanned literally hundreds of different species,’ he says. ‘Every fossil ever found has suddenly got a new lease of life.’
Structural colour
The recent explosion of interest in fossil colour has been dominated by the search for pigments. But there are other important contributors to colour in organisms. Some of nature’s most vibrant visual effects are produced by nanoscale structural features interacting with light – such as the iridescent sheen of beetle carapaces or peacock feathers.
It is these structural colours that interest Maria McNamara, a geologist at University College Cork in Ireland, whose work has focused primarily on fossilised insects. Insect structural colours come from multilayer reflectors in their protein- and lipid-rich cuticle, which contain alternating layers of materials with different refractive indexes. These produce bright metallic colours by causing interference in the reflected light waves.
‘Many fossil insects show bright metallic colours – greens, blues, reds – and [under the SEM we find] these tiny multilayer structures preserved in the outer coating,’ says McNamara. ‘But the structures that are preserved actually can’t produce the colours we see today – something has changed about them during fossilisation.’

McNamara is trying to unpick these changes so that the bugs’ original colour can be determined. She does this by making her own fossils from dead beetles. ‘During fossilisation these things are being squashed and heated up, and this “cooking” process is what drives the physical and chemical changes,’ she says. So McNamara ‘cooks’ her specimens in a purpose-built autoclave at temperatures up to 300°C and pressures up to 500 atmospheres to simulate conditions deep in the Earth’s crust. She can then use GC-MS to monitor their chemistry.
The visible colour changes during these experiments are striking, with the beetles’ cuticle colour being dramatically blue-shifted over just 24 hours.6 This is all down to chemical transformations in the multilayer reflectors, according to McNamara.
‘You start off with lots of fatty acids, but putting them into the experiment generates a lot of complex molecules, which change their refractive index,’ she says. This in turn changes the way the reflectors interfere with light and ultimately the colour they produce. The group are now trying to work backwards and develop a formula that can be applied to fossil insects to deduce their original colour using a combination of electron microscopy and chemical analysis.
Our job is to bring the past to life, and we can now do it a bit better
Mike Benton
Like many working in the field, McNamara has recently turned her attention to feathers. In modern birds, bright colours come from structures made of keratin, the protein that makes up the bulk of the feather, interacting with layers of melanin, and occasionally other pigments. Unfortunately, keratin disappears during fossilisation, so any evidence of these structures is unlikely to survive. So far the only suggestion of structural colour in a feathered dinosaur has been a distinctive arrangement of long, thin rectangular melanosomes in a microraptor that hinted at iridescence.7
McNamara’s autoclave experiments are helping to unravel what happens to more complex colours by examining keratin and melanin degradation in modern feathers. Her group recently showed that while melanosomes are resilient, their shape and structure can change significantly under high heat and pressure.8 Does this leave a question mark over earlier studies that assume the melanosome morphology in fossils is unchanged? Perhaps, says McNamara, but making sense of these hugely complicated processes is an important piece of the puzzle.
The future of the past
It’s still early days for the study of colour in palaeontology, and in some ways every discovery raises more questions than it answers, says Manning. ‘Colour is complicated – it is not a one-hit wonder that we can diagnose from a single variable,’ he says. ‘I think we’ve got to spend the next few years hunting down biomarkers for the different types of dominant pigment. Each one is like another dollop on your palette of colour for the fossil record.’
But he is optimistic for the future of this rapidly growing field: ‘I think the right people are doing the right things at the moment, and eventually the research will come together.’
Benton shares this positive outlook, and reflects that huge progress has already been made. ‘Our job is to bring the past to life, and we can now do it a bit better,’ he says. ‘There’s probably more than a hundred [fossils] whose colour we can reconstruct. 10 years ago we would have said this was impossible.’
References
1 F Zhang et al, Nature, 2010, 343, 1075 (DOI: 10.1038/nature08740)
2 Q Li et al, Science, 2010, 327, 1369 (DOI: 10.1126/science.118629)
3 K Glass et al, Proc. Natl. Acad. Sci. USA, 2012, 109, 10218 (DOI: 10.1073/pnas.1118448109)
4 P L Manning et al, J. Anal. At. Spectrom., 2013, 28, 1024 (DOI: 10.1039/c3ja50077b)
5 R M Carney et al, Nat. Commun., 2012, 3, 637 (DOI: 10.1038/ncomms1642)
6 M E McNamara et al, Geology, 2013, DOI: 10.1130/G33836.1
7 Q Li et al, Science, 2012, 335, 1215 (DOI: 10.1126/science.1213780)
8 M E McNamara et al, Biol. Lett., 2013, DOI: 10.1098/rsbl.2013.0184












No comments yet