With a rumble like a long roll of thunder, a lorry tips 28 tonnes of apples into a concrete pit in just a few seconds. The air they displace engulfs onlookers, enveloping them in an intensely sweet, fruity aroma that Thatchers, the UK cider maker based in Somerset, hopes will eventually reach its customers.

While turning apple sugars to alcohol is the best known part of cider production, controlling the aromas and flavours that come with it is just as, if not more, important. Richard Johnson, Thatchers’ quality manager, thinks he and his colleagues do that well. ‘We make 50–60 million litres of cider each year currently, growing every year,’ he says. ‘People seem to like what we do, so they keep buying more.’
Thatchers isn’t the only one riding high on the cider wave – production across the whole UK has increased five-fold since the 1970s.1 And in recent years, alongside industrial companies there’s also been a resurgence at smaller scales, reviving traditional farmhouse ciders.
Whatever cider you prefer, the secrets of success lie in how apple compounds are processed by people and transformed by yeasts and bacteria. Chemists have therefore been drawn to cider at least since the ‘father of modern chemistry’ Antoine Lavoisier’s day (see box) in the 18th century. Many people today know and love the fruits of their labour – but even now, research is still ongoing to create a tastier tipple.
Cider secrets
To make cider, apples should be fully ripe, with any starch they contain fully broken down into sugar, ready to become alcohol. Thatchers therefore dips samples of its growers’ apples in iodine, paying them ‘a substantial bonus’ if rather than turning black, indicating the presence of starch, they remain unchanged, indicating sugar. As the sample is being tested, workers use water to wash the rest of the load along a canal at the base of the apple pit into a mill. ‘Rotten apples won’t float, they sink, as does any stone or anything that’s come in with them, so only good, clean fruit go into the process,’ Johnson explains.
The mill mashes and feeds the apples into a press, where a piston crushes them and forces juice out into a tank. Often overlooked, this is a key stage in cider production, where enzymatic processes affecting the final drink begin. Lipoxygenase enzymes start converting fatty acids to aldehyde aroma precursor chemicals, while polyphenol oxidase drives the darkening that gives apple pulp and juice its golden colour. Oxidised apple tannins can stick to the pulp, depriving the final cider of their flavour. ‘Anybody who’s bitten an apple will know that it can turn brown very quickly, so we turn apples into juice and cider as fast as possible,’??Johnson?says.
Today, UK producers like Thatchers concentrate and store their own juice so that it’s available to make cider with all year round, according to independent cider industry chemist Andrew Lea. Most will then strive to ferment all the apple sugar to 10–12% alcohol in as little as two weeks, diluting it before bottling or kegging.
Extending this approach has brought about a current trend at other manufacturers towards cider with the flavour of pear and other fruits. ‘Because alcopops [sweet, fruit-flavoured drinks] got a bad name, that market has been taken over by fruit-flavoured ciders,’ Lea reveals. ‘You can buy cheap apple juice concentrate and make cider without a lot of trouble, and then they have fruit flavours added.’
By contrast, smaller producers tend to use fresh juice, fermenting it for three to four months to get a more complex taste profile. And while yeast and bacteria in industrial fermentations are carefully controlled, smaller producers often exploit microorganisms naturally present on the apples and in the air when searching for flavour. But when it comes to fizziness, producers large and small usually rely on injecting carbon dioxide to finished cider rather than bottle conditioning, where gas produced during fermentation is trapped.
The clarity of their products is also more similar than some people might imagine, Lea says. ‘There’s a myth that cloudy cider is more natural than clear cider, but a lot of ciders drop bright and clear anyway, even on a craft basis. It has absolutely no impact on how nutritious or alcoholic the cider is, or whether it rots your guts. The cloud could be anything, pectin, polyphenols, yeast. Most industrial ciders are filtered but sometimes they’re clouded deliberately to suit the product style.’ At Thatchers, juice flows through overhead pipes into stainless steel tanks for the fermentation that turns sugar to alcohol. The temperature is carefully controlled, Johnson emphasises. ‘That really gives us consistent quality batch after batch after batch,’ he says. The company uses yeast that multiplies rapidly and outcompetes other microorganisms, avoiding the sodium metabisulfite typically used in homebrewers’ Campden tablets to kill bacteria that would produce acetic and lactic acid. To make the fermentation quick, industrial cider makers also feed nutrients such as ammonium salts and vitamins into their tanks.
Once the cider has fermented, for many Thatchers brands it is transferred into and then matured in vats made of centuries-old oak. Throughout all the company’s processes, Johnson’s lab team performs regular tests on yeast health during fermentation and more generally on acidity and alcohol levels, colour and clarity. They also use headspace gas chromatography, where an inlet positioned above a sample of cider can extract key flavour and aroma compounds for measurement.
Yet Johnson and the rest of Thatchers’ taste panel must still sample every vat in the factory at least once a week, which means six to 10 ciders a day. ‘You can’t ever analyse as well as your nose and tongue can,’ Johnson says. ‘We swallow to get maximum flavour, because a lot of what you think is taste is actually smell. That comes up the back of your throat and into your nasal cavity, so you do get a better appraisal of flavour by actually drinking the product.’
Polyphenolic spree
The apples used to make the juice determine the non-volatile components of the final cider flavour. While Thatchers has a museum orchard with 458 varieties of apple, it currently uses 25 commercially, with varying flavours determined mainly by levels of tannins and malic acid. ‘There is a formal classification of cider apples into four main groups: bitter-sweets, bitter-sharps, sharps and sweets,’ says Lea. ‘A heavy bitter-sweet apple has a heck of a lot of tannin and very little acid, whereas a dessert apple will have bugger all tannin and a great deal of acid. If your acid level is too high or too low it’s unpalatable. Getting the best level in the final product is all a matter of blending.’
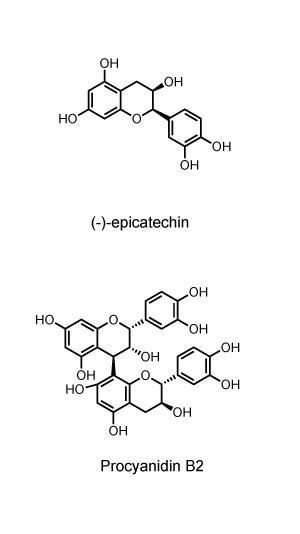
In the 1970s, Lea helped unravel the specific chemical structures of apple tannins.2 A subset of the flavonoid group of compounds, they’re oligo- and polyphenols mainly based on the building block (–)epicatechin. Typically several (–)epicatechin units will be linked together to produce a range of oligomers known as?procyanidins.
‘We established that the difference between bitterness and astringency was largely to do with the degree of polymerisation of the procyanidins,’ Lea recalls. With two to four (–)epicatechin units procyanidins are more bitter, whereas with five to seven units they’re more astringent. Some bitter taste is desirable, whereas a mouth-puckering astringency is less welcome. Thatcher matures its ciders for smoothness because Somerset cider apples have high tannin levels. ‘The modern consumer doesn’t like high tannin much,’ Lea says.
Volatile flavour compounds like esters, by contrast, come from the alcoholic fermentation, after which UK industrial producers often make sweet ciders by adding sugar or artificial sweeteners. Smaller producers, however, might exploit an additional malolactic fermentation stage that turns malic acid to lactic acid, reducing acidity levels and bringing other flavours.
‘There’s a whole range of volatile substituted phenols that are produced by malolactic bacteria that give what is known as an old horse or farmyard note,’ Lea says. ‘At a certain level those are very desirable in the cider, but over a particular threshold they are regarded as an off flavour.’ Other volatile compounds indicate unwanted microbiological activity, with ethyl acetate, which most people identify as a vinegary smell, amongst the most notable.
Cidre, not cider
In France, the general distinction between large and small scale producers in control over microbes and their effects is similar to the UK. That’s what Jean-Michel Le Quéré from the French National Institute for Agricultural Research (INRA) in Rennes and his colleagues found after studying 90 French ciders.3 From 2004–2006, Le Quéré’s team tested ciders using both an expert tasting panel and chromatography, and also asked producers about their processes.
‘Industrial ciders appear more aromatic but with a jam aroma, which is probably due to heat treatments,’ Le Quéré says. ‘Farmers’ ciders are more often bitter, astringent and sometimes contain volatile phenolic compounds due to Brettanomyces yeast. This characteristic is now less accepted by consumers and most small producers are trying to reduce it.’

This year, the INRA and the French Institute of Cider Production (IFPC) in Le Rheu published the results of revisiting Lea’s 1970s work on procyanidins.4 Their study focused on how the compounds’ role is affected by the French practice of intentionally starving yeast of nutrients to stop fermentation before completion, keeping the cider sweet. They showed that procyanidins reduced perceived sweetness and increased both bitterness and astringency, with the level of polymerisation playing a less important role.
Le Quéré highlights that volatile compounds also potentially play a key role in French cider’s chemical characteristics, but that knowledge of that role is ‘rather poor’. Consequently he and his colleagues have teamed up with Angélique Villière from the flavour group at ONIRIS, the Nantes Atlantic College of Veterinary Medicine, Food Science and Engineering. Having noticed that previous studies of cider’s volatile components didn’t explore how those compounds affected cider’s odour, Villière and her colleagues developed a technique that could do this.5
‘We have a chosen a method to extract volatile compounds that matches as closely as possible the odour of cider,’ Villière explains. Before performing any analysis on cider odour compounds, those compounds have to be extracted, and often extraction methods disproportionately collect more or less volatile compounds. For the most balanced extraction, the ONIRIS team chose solid phase microextraction, holding carboxy-polydimethylsiloxane fibre adsorbents over their cider samples, keeping them at 37°C.
After extracting odour samples, Villière’s team analysed them using familiar chemical techniques of gas chromatography–mass spectrometry, coupled with the less common method known as olfactometry. In this approach, a team of trained experts use their noses to identify each smell as they elute from the gas chromatograph. ‘It’s complicated because you smell the odour for only one to five seconds, so you have to be able identify it quickly,’ Villière underlines.
While the key cider aroma chemicals they identified – mainly esters and long-chain alcohols – held few surprises, Villière stresses the study’s importance lies in the technique’s usefulness to producers. Villière and Le Quéré are now using the approach together to look at how cider production processes influence volatile compound profiles, similarly to Le Quéré’s 2004–2006 study.
A cider a day?
Beyond their role in cider flavour, (–)epicatechin and procyanidins have recently been gaining media attention because of their potential health effects. Scientists have identified flavonoid polyphenols such as tannins as possibly playing beneficial nutritional roles, for example in wine. Though they have been thought to serve as antioxidants, the amount that enters the bloodstream seems too little for that to be the case. Current theories suggest that the compounds may play a role in regulating gene expression.
Many of the studies into the effects of these compounds have been funded by industries whose products contain them, particularly chocolate makers. But from 2003–2007 the UK National Association of Cidermakers sponsored a number of projects, confirming to what degree these compounds are present in cider.6Alan Crozier, now at the University of California, Davis in the US, whose team did that work, underlines that procyanidins’ and (–)epicatechin’s bioavailability might be underestimated. ‘If you look at what’s been excreted you get a clearer view of what’s been absorbed,’ he explains. ‘Colonic microflora break down flavonoids to low molecular weight phenolic acids, and if you start to take into account some of the smaller metabolites then?most of these things are highly?bioavailable.’
But Crozier is quick to stress that there’s no evidence that this makes cider good for us. ‘The work hasn’t been done, it’s as simple as that,’ he points out. ‘And let’s be frank, when there’s alcohol in there I doubt that there’s really any serious health benefits other than a relaxing glass in moderation rather than excess.’
While that’s perhaps a little disappointing, knowing that there’s still so much molecular tinkering possible presents chemists with temptations that blur the private and professional. Does one drink cider crafted by experts like Thatchers or accept the microbiological challenge of making it at home – or possibly even both?
Lavoisier’s liquid legacy

In the 18th century, both sides of the Channel saw cider cause mass lead poisonings. In the UK, the ‘Devon colic’ arose from lead-lined vats and pipes. In France, cider made in Normandy was clarified with ‘white lead’, a mineral comprising lead hydroxide and carbonate.7 The Norman parliament banned this practice, instituting a test based on foie de soufre volatil. That translates directly as volatile liver of sulfur, an apt term for sulfurated potash, a poorly defined mixture of potassium sulfide and other compounds of potassium and sulfur. If lead was present, this compound would produce an easily identified black lead sulfide precipitate.
However, cider makers were also adding chalk, which was in itself considered dangerous, and also thought to render the liver of sulfur test ineffective. A second test was instituted, where adding ‘fixed alkali’, solid sodium or potassium carbonate, was thought to indicate chalk was present if a precipitate formed. Yet many French scientists doubted this test worked, causing a row that eventually forced the Royal Academy of Sciences to intervene. They called on a dream team of 18th century French chemists: Jean d’Arcet, Louis Claude Cadet de Gassicourt, Antoine Baumé, Claude Louis Berthollet and Antoine Lavoisier. The five set up a cider press in Baumé’s chateau, producing a drink whose quality Lavoisier rated as poor. Nevertheless they were able to show both that the chalk test didn’t work, and that chalk didn’t stop the liver of sulfur test?working.’
Andy Extance is a science writer based in Exeter, UK
References
- A G H Lea, Oxford handbook of food fermentations, Oxford University Press, 2014
- A G H Lea and G M Arnold, J. Sci. Food Agr., 1978, 29, 478 (DOI: 10.1002/jsfa.2740290512)
- J-M Le Quéré et al, LWT – Food Sci. Technol., 2006, 39, 1033 (DOI: 10.1016/j.lwt.2006.02.018)
- R Symoneaux et al, LWT – Food Sci. Technol., 2014, 57, 22 (DOI: 10.1016/j.lwt.2013.11.016)
- A Villière et al, Food Chem., 2012, 131, 1561 (DOI: 10.1016/j.foodchem.2011.10.008)
- S C Marks et al, J. Agric. Food Chem., 2007, 55, 8723 (DOI: 10.1021/jf071155u)
- O Lafont, Hist. Sci. Med., 1996, 30, 31





















No comments yet