Sophisticated analytical chemistry is studying our neighbouring planet, as Andy Extance discovers
This chance encounter is one of several pieces of evidence that emerged in the early 21st century suggesting Mars could once have supported life. In 2002, Nasa’s Mars Odyssey orbiter found large subsurface water ice deposits, supporting the idea that the red planet once had lots of liquid water near the surface.A decade ago, travelling across Mars’ dusty, mostly frozen, surface to examine its own discarded heat shield, Nasa’s Opportunity rover made an exciting discovery: a basketball-sized iron–nickel meteorite. Today, this type and size of meteorite would vaporise on impact. To exist, it must have been slowed down by a past atmosphere, thicker and warmer, which has since decayed to today’s thin, carbon dioxide-dominated state.

‘Some people have suggested that life started on Mars and came to Earth,’ says geochemist Jennifer Blank, a senior scientist at the Nasa Ames Research Center in California, US. ‘In the first half billion years of our solar system’s history, Mars had water and was warmer.’ At that time, around four billion years ago, Earth was still inhospitable, with a partially molten surface, while both Earth and Mars were also being bombarded by meteorites. ‘Mars in the beginning must have been pretty wet, and on Earth life arose very soon after it was possible to survive,’ explains Fred Goesmann from the Max Planck Institute for Solar System Research in Göttingen, Germany. ‘It would be interesting even if there is nothing living on Mars – we’d want to find out why not.’
Among many implications from these findings, one is that in the last four billion years something very bad must have happened to Mars’ atmosphere – but what? And if it seems possible that life may once have existed there, could it still? That prospect seemed even more plausible when, in 2003 and 2004, a series of orbiters and Earth-bound telescopes reported plentiful methane on Mars. With most methane on our planet produced by living organisms, could the same be true of our neighbour?
These mysteries all helped set the scientific agenda for current Mars missions, in particular Nasa’s Curiosity rover. Curiosity has now clocked up more than a thousand Martian days, confirming that the general prerequisites for life have been present on the red planet. But it’s also highlighted a new set of puzzles that the next generation of Mars missions will seek to solve.
Caught on camera
Since dramatically descending to Gale Crater on 6 August 2012, Curiosity has been guided by the ChemCam instrument that Blank calls its ‘chemistry scout’. Blank works planning activities as a ‘science uplink’ for ChemCam, and as a ‘downlink’, interpreting potentially huge numbers of spectra returning. ‘ChemCam can fire a thousand shots in one day, it’s fast and consumes little energy,’ she explains. ‘As a result it’s used a lot. The laser makes a tiny plasma and a camera looks at light emission that occurs when the plasma’s cooling down.’

Thanks to its guiding role, ChemCam was the first Curiosity instrument to identify water in the planet’s soil. ‘One element that has a strong signal with ChemCam is hydrogen,’ Blank says. ‘At the surface, all targets have high hydrogen absorption.’ As Curiosity went to and paused at a sandy location dubbed Rocknest, ChemCam consistently found around 2% water by weight in 139 targets.1 ‘There’s a lot more water than in lunar rocks,’ Blank observes. ‘But in terms of extracting it, you’d have the same challenges as extracting water from Earth rocks.’
But ChemCam’s particular sensitivity to hydrogen and other elements like iron and magnesium mean these elements’ spectra can overwhelm others. ‘I’m looking for carbon and phosphorus in my role too, and it’s challenging,’ Blank admits. Thankfully, after ChemCam’s scouting efforts, Curiosity can call on other instruments. At Rocknest, Curiosity got a chance to reach out its robot arm to scoop up samples – and in the process stirred up yet another Martian mystery.
The crystals that weren’t there
Rocknest’s was the first Martian soil analysed by x-ray diffraction, in Curiosity’s CheMin instrument. CheMin can identify most minerals, says David Vaniman from the Planetary Science Institute in Tucson, US, who is the deputy principal investigator for the instrument. ‘Perhaps the most important aspect of CheMin analyses is determination of full mineral assemblages,’ Vaniman explains. ‘This is usually not possible with remote sensing. CheMin measures all crystalline minerals in a sample, and can determine which are not present. This is important because minerals that occur together can indicate, for example, the composition of mineral-precipitating or mineral-altering fluids.’
In fact, there was a lot more ‘not present’ than anyone expected. Around 30–45% of the Rocknest scoop didn’t give any x-ray diffraction pattern,2 a finding that has since been repeated in other locations. Curiosity’s team is still working to interpret this amorphous material.
The scooped Rocknest sample also gave the Sample Analysis at Mars, or Sam, instrument a chance to flex its scientific muscles, wringing important conclusions from the water residues. Sam contains a furnace that can heat samples to 900°C, monitoring the gases released by gas chromatography–mass spectrometry (GC–MS) and with a tuneable laser spectrometer (TLS). Water was among the gases released at Rocknest, explains Paul Mahaffy, the chief of the Planetary Environments Laboratory at Nasa Goddard Space Flight Center in Maryland, US.
Sam later found that a mudstone rock encountered on Mars contained 1.5–3% water, but more importantly, it also identified that water’s origin. The clues came thanks to Sam’s TLS, which can measure the ratio of deuterium to hydrogen (D/H).3 ‘Water released at lower temperatures came from the atmosphere,’ Mahaffy says. ‘Its D/H ratio is close to the atmospheric value and so this water must exchange with the atmosphere. Water released at temperatures above 550°C retains a much lower D/H ratio, preserved from more than three billion years ago. Since then, more hydrogen than deuterium was lost from the atmosphere and what was left became enriched in deuterium.’
Sam has also seen this loss of lighter isotopes, or fractionation, in carbon dioxide, nitrogen, argon and xenon, indicating the atmosphere’s steady top-down loss. The process and rate of gas escape is also currently being studied by Nasa’s Mars Atmosphere and Volatile Evolution (Maven) probe, whose elliptical orbit takes measurements at varying heights. A Nasa announcement in December 2014 hinted that the orbiter has observed evidence of the process starting with high-energy particles from the Sun acting on upper atmosphere gases.
Safe harbour
Knowing there is water on Mars, the question becomes whether it could support life. Images from Nasa’s Mars Reconnaissance Orbiter had apparently shown a former lake, where life might have existed, in Gale Crater at a site called Yellowknife Bay. From Rocknest, Curiosity proceeded to Yellowknife Bay to drill its stones, finding they were in fact formed from ancient mud.
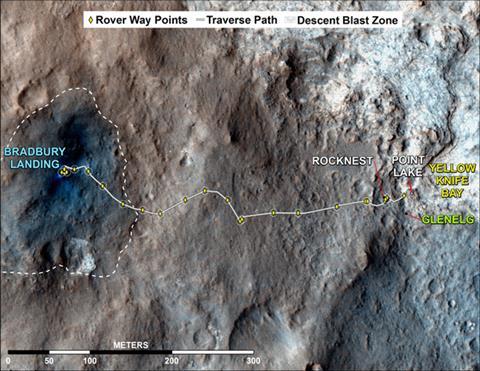
‘One goal was to determine if clay minerals were present, and if so whether they were products of acidic or high-temperature alteration or more benign conditions,’ Vaniman explains. ‘At Yellowknife Bay, clay minerals are a key component and CheMin determined that they are smectites consistent with formation below 75°C, a temperature range where life would be possible. The samples were notably lacking some minerals observed elsewhere on Mars, such as iron sulfates that indicate acidic water. The only sulfate minerals present at Yellowknife Bay were calcium sulfates more consistent with precipitation from near-neutral solutions.’
CheMin also found that the minerals contained sulfur and iron in different oxidation states.4 ‘These could be used by organisms that require either oxidation or reduction to support metabolism,’ Vaniman suggests. Sam and Curiosity’s alpha particle x-ray spectrometer have found each key element for life – carbon, hydrogen, oxygen, phosphorus and nitrogen – meaning Yellowknife Bay satisfies the basic conditions for habitability.
But stronger evidence for life on Mars remains elusive. Notably, Sam has found typically just one part per billion (ppb) of methane in the atmosphere, with no evidence of it being produced by microbes. Then, after Curiosity had spent more than a year on the surface, that level spiked up to around 7ppb, staying there for two months before subsiding again.5 ‘Why does methane come and go?’ Mahaffy asks. ‘We don’t understand this yet.’
Curiouser and curiouser
The search for more complex organic molecules that living organisms might have produced may be stymied by a phenomenon linked to odd observations dating back to the 1970s. Then, the two Nasa Viking landers detected chloromethane and dichloromethane, at the time dismissed as residual cleaning solvents used on Earth. Their results also suggested the planet’s surface was covered in a highly oxidising compound, hydrogen peroxide, that would destroy organic chemicals rapidly. However, in 2009 Nasa’s Phoenix lander found extremely oxidising perchlorate salts – valued for use as rocket propellants on Earth – near Mars’ North Pole. Since then, some scientists have performed experiments that suggest reactions between organics and perchlorates could have made the chloromethane and dichloromethane Viking detected.6
Sam’s measurements add to growing evidence that perchlorate salts are ubiquitous on Mars’ surface, creating an extremely harsh modern environment that poses serious challenges to life. In April 2015, Nasa scientists, working from Curiosity’s temperature and humidity measurements, suggested these salts could even sometimes help liquid brines form in soil at night.7 They could absorb water and lower its freezing temperature enough to explain ‘dark flows’ seen on slopes by the Mars Reconnaissance Orbiter in warm seasons.
This underscores the challenges of analytical chemistry on the surface of another planet
This phenomenon offers a potential explanation for the mysterious amorphous component CheMin found amongst Mars’ dust, Blank suggests. ‘There have been discussions about whether these brines help weather away surface material over millennia and then create this amorphous phase,’ she says. ‘To me that’s the most appealing hypothesis, that long-term low-temperature surface operation.’
Although Sam has detected some organic compounds by its usual heating method, that heating can accelerate perchlorate oxidation, obliterating them. To overcome such problems, Sam co-investigator Goesmann explains, the instrument carried limited amounts of ‘strange chemicals that can attach wings to molecules’, better known as derivitisation reagents. Intended to separate polar organic molecules like amino acids that are more likely to break down than evaporate, one such liquid has inadvertently featured more prominently – because it leaked.
‘With our very first GC–MS experiment we saw reaction products of our wet chemistry agent,’ Mahaffy explains. ‘We are quite effectively working around this terrestrial vapour and also now allowing it to do what it was designed to. This is different from the original design of our experiment but it works and we are detecting more organic compounds than would be possible without these reagents. But this just underscores the challenges of implementing complicated analytical chemistry techniques on the surface of another planet.’
The quest for organics
While Curiosity strives to overcome these difficulties, the next generation of Mars missions is designing instruments with them in mind. Goesmann is principal investigator for the Mars Organics Molecule Analyser (Moma) instrument, the largest on the joint European–Russian ExoMars rover mission due to launch in 2018. Moma will include both GC–MS (with derivitisation reagents similar to Sam’s) and laser desorption mass spectrometry.
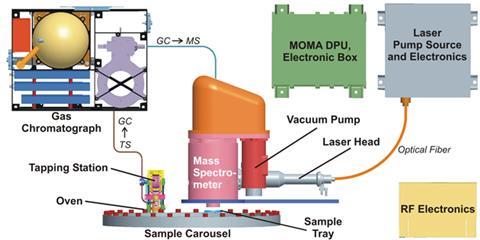
‘Laser ionisation is so quick that you have these molecules in the gas phase before they can react with anything, and you can look at very heavy molecules,’ Goesmann explains. ‘The disadvantage being that you look at everything at the same time, you can’t separate out the molecules. That’s why we want to fly this combination.’ He and his colleagues have already tried using the technique with perchlorates present, finding that many organic molecules can survive.
In anticipation of successfully extracting organic molecules, Moma is being equipped with GC capabilities that can separate mirror-image isomers. ‘The underlying idea is to find patterns,’ Goesmann says. ‘If you’re just doing statistical chemistry, mix reagents, make them react, there’s no particular preference for chirality. If there’s no enantiomeric excess, that would mean it’s a bit boring, probably statistical. If there is a strong preference, what causes this break in symmetry? Biology is strongly biased, your chemistry lab is not.’
The ExoMars mission will also be the first to send an instrument exploiting Raman spectroscopy to a planetary surface for remote analysis, highlights Howell Edwards from the University of Bradford. Edwards and the University of Leicester’s Ian Hutchinson are heading the UK team behind the detector and electronics for the Raman laser spectrometer (RLS). The tool will figure prominently in ExoMars’s efforts to protect against the oxidising surface environment. ‘A novel aspect of the mission is the drill, which will bring samples from about two metres below the surface for analysis,’ Edwards says. Non-destructive and suiting both organic and inorganic analysis, RLS will be the ‘first pass molecular interrogation technique’ for the material retrieved, before it passes to other ExoMars instruments.
That’s quite a different approach from Nasa’s next rover mission, Mars 2020. Mars 2020 will also feature a Raman instrument, named Scanning Habitable Environments with Raman and Luminescence for Organics and Chemicals, or Sherloc. Sherloc is intended to analyse surface minerals and potential biogeological samples, specifically targeting carbon ring compounds.
If successful, these instruments will bring humanity nearer to answering the question that’s long fascinated us – just how unique is life on Earth? But for now, Goesmann is focussing on a simpler question. ‘Trying to find life is a big thing,’ he says. ‘If it’s a hamster jumping up and down, OK, we found life, but I’d rather say we’re trying to find organic molecules. At the surface they’ve probably been oxidised but further down they could have been embedded somewhere. Finding organics and patterns in them, I would be happy with that.’
References
1 P-Y Meslin et al, Science, 2013, 341, 1238670 (DOI: 10.1126/science.1238670)
2 D F Blake et al, Science, 2013, 341, 1239505 (DOI: 10.1126/science.1239505)
3 C R Webster et al, Science, 2013, 341, 260 (DOI: 10.1126/science.1237961)
4 D T Vaniman et al, Science, 2014, 343, 1243480 (DOI: 10.1126/science.1243480)
5 C R Webster et al, Science, 2015, 347, 415 (DOI: 10.1126/science.1261713)
6 F J Martin-Torres et al, Nat. Geosci., 2015, 8, 357 (DOI: 10.1038/ngeo2412)
7 R Navarro-Gonzalez et al, J. Geophys. Res., 2010, 115, E12010, (DOI: 10.1029/2010je003599)












No comments yet