Rachel Brazil talks to the scientists trying to recreate what the first cells were like, or to make their own versions
How did the first cells develop from a prebiotic soup of chemicals, probably around three and a half to four billion years ago? ‘Biologists often don’t think about it, even though they know more about the cell than any of us,’ says Stephen Mann, chemist and director of the centre for protolife research at the University of Bristol, UK. It’s been largely left to chemists to probe how early cellular life came into being. Ever since the famous Miller–Urey experiment in 1952, they have shown how some of the basic chemical building blocks of life might have been synthesised from simple organic molecules, but there is still a huge gap between this and the existence of replicating cells and biological life.
Origin-of-life researchers are hoping to plug that gap by studying protocells. ‘We think protocells can be the potential path between non-living and living systems,’ says Yan Qiao, a chemist at the Chinese Academy of Sciences in Beijing, China. Protocells are cell-like compartments that attempt to mimic the earliest stages and functionality of cellular life. ’When we think about what makes something a protocell, rather than it just being a droplet or a sack of lipids, it should make use of energy, be able to grow and divide … and the most importantly, transmit information to its future generations,’ says biophysicist Anna Wang from the University of New South Wales in Australia. ‘No one’s been able to do all of those bits and piecest simultaneously in a lab yet, especially using materials that could have existed on early Earth,’ she adds.
To be plausible, protocells need to be compatible with modern biology. ‘It’s important to be able to conceive of that continuity,’ says Wang. That leads to the question of whether the first cell-like compartments could have started with the phospholipid membranes we see in cells today. ‘That would be a problem,’ says Mann. ‘Because such membranes are rather impermeable, so you can’t get really get much through them.’
An idea initially put forward by Soviet biochemist Alexander Operin in the 1930s suggested that the first cells were phase-separated droplets without membranes, known as coacervates. Operin carried out some pioneering research on the origins of life, although his 1938 book on the topic received a sceptical review in Nature, including criticism of his frequent quotes from Friedrich Engels.
Coaxing droplets
In the last decade Mann has revived this idea and has opened up research into coacervates. They appear when two oppositely charged polyelectrolytes come together in solution, forming a separate condensed phase of highly enriched droplets, and often attracting other small molecules into the phase. ‘The polyelectrote can be RNA, DNA, proteins, peptides or polysaccharides like dextran sulfate,’ explains Qiao. The process is very fast, spontaneous and they form in a broad range of chemical conditions.
Qiao points out that cells contain sub-compartments or organelles that, in common with coacervates, do not have membranes. Proteins are assembled in ribosomes, for example, which are essentially bicondensates of RNA and proteins. ‘We know that RNA can also be used to catalyse chemical reactions in cells and so if a protocell looked like a ribosome, it could be used to [catalyse] chemical reactions and also to pass genetic information to offspring,’ suggests Qiao.
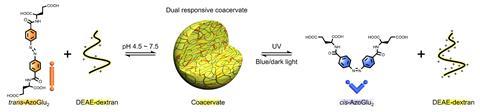
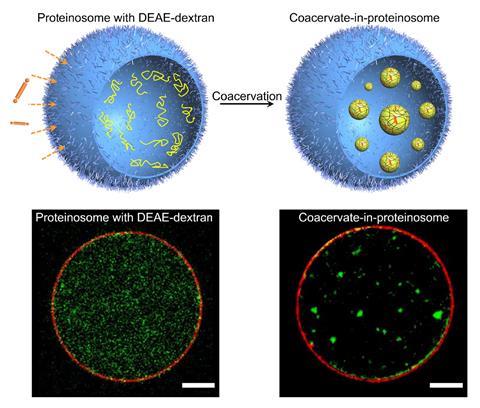
She has been trying to mimic cell organelle formation by creating coacervates inside a synthetic semi-permeable vesicle known as a proteinosomes, originally developed in Mann’s Bristol laboratory. These are made from a closely packed monolayer of the protein – bovine serum albumin in this case – and polyamides, and they spontaneously self-assemble. Qiao formed a coacervate by initially loading the proteinosome with the cationic polyelectrolye diethylaminoethyl–dextran, and outside added an azobenzene molecule, substituted with two negatively charged glutamic acid groups. This molecule was able to permeate the membrane, forming a coacervate inside. She also found that if a single-stranded DNA molecule was introduced, it would become partitioned into the coacervate.
Qiao was able to use her coacervate protocell to process binary information – perhaps the precursor to the complex signalling cascades that modern cells perform. Making use of the photoresponsive azobenzene molecule and the pH-sensitive nature of both molecules, she was able to use both UV light and changes in pH to disassemble the coacervate inside the protocell. The change from the cis to trans configuration that occurs in azobenzenes on illumination with UV light reduced the attraction between the two polyelectrolytes, as did an increase in pH, both causing the coacervate to collapse. By introducing various enzymes, she was also able to create chemical signalling, so that adding urea or a combination of lactose and oxygen would cause the coacervate to dissolve.
So could coacervates represent the first protocells – something like a ribosome, with the cell membrane coming later? Not everyone is convinced. While they form easily, coacervates also spontaneously coalesce and fuse together. ‘They are inherently unstable,’ says Mann. He points out that one of the major features of living cells is their ability to harness energy, taking advantage of chemical gradients either side of their membranes. ‘Coacervates are problematic in that regard because, with no membrane, they are in open equilibrium with the environment.’
Soapy parcels
Others are pursuing an alternative model for protocells based on self-assembled fatty acid vesicles – essentially the sort of surfactant molecules used to make soap. ‘They can make these quite dynamic and interesting membrane mimics,’ says Wang, ‘but they’re certainly not the membranes that we find in modern organisms.’ But they do provide a means of creating a delineated compartment, unlike coacervates. Deciding which model is more likely has seen chemists trying to mimic some of the properties found in living cells – such as an ability to grow and divide.
‘The acquisition of the proliferative ability of protocells is the missing link between “chemical evolution” and “Darwinian evolution”,’ says Muneyuki Matsuo, a biochemist and an origin-of-life researcher at Hiroshima University in Japan. It has been high up on the list of attributes the protocell would need to have. Wang has examined the properties and conditions need for fatty acid vesicles to grow and divide. She found one method was to start with two populations of vesicles with multiple or single lipid layers and each made from different length fatty acids, from eight to 18 carbons.
’When you put two vesicles that are made of different lipids near each other, they’re going to start exchanging material… sometimes it’s dramatic enough that you can get some of them to cannibalise all the lipids off the other type of vesicle, [which] dramatically increases in surface area, [and this] can be really destabilising and lead to division,’ explains Wang. But whether this really constitutes growth is debatable. ‘The growth step is from some sort of reservoir of material,’ she admits.
Another challenge with protocells made from fatty acid vesicles is the route to cells containing information-carrying molecules such as RNA and DNA. A large proportion of origin-of-life researchers support the RNA world hypothesis which suggests that, before DNA, the first self-replicating, information-carrying molecule was RNA. As well as their role in protein synthesis, RNA-based catalysts called ribozymes (ribonucleic acid enzymes) are still involved in cellular processes including RNA splicing in gene expression, so the RNA world proposes that self-replicating RNA molecules existed before protein synthesis took over.
Thioesters may have been the energy currency of primitive metabolism
The problem with this idea is that ribozymes need the assistance of magnesium ions to function. The ion stabilises intermediate structures, allowing the RNA molecule to fold into the correct conformation. But magnesium ions also disrupt fatty acid membranes. The group of Jack Szostak at the Harvard Medical School in Boston, US, has been examining this problem. The 2009 physiology Nobel prize winner now focuses his research on understanding the origins of life and has tried to bypass this problem using citrate-chelated magnesium(II), which is able to assist in the templated copying of RNA using trimers of 2-aminoimidazoyl-activated RNA. Adding the aminoimidazoyl leaving group on the nucleotide phosphate group activates the RNA extension.
Groups working on the alternative coacervate model are also experimenting with proliferation and growth. In 2021 Matsuo and collaborator Kensuke Kurihara from the Exploration Research Center on Life and Living Systems (ExCELLS) in Okazaki, Japan, showed that coacervate droplets could also proliferate through peptide production and self-assembly. They used an amino acid thioester monomer, which formed peptides and concentrated in droplets. ‘In the current coacervate system, monomers are concentrated in the peptide-based droplets by intermolecular interactions, making the reaction autocatalytic and self-reproducing,’ explains Matsuo; this leads to the growth of the droplets and eventual division. The droplets were also able to concentrate other macromolecules such as nucleic acids.
The amino acid thioester structure used was inspired by the hypothesis that thioesters were probably abundant in prebiotic chemistry and, given the high energy of the thioester bond, they could have provided the basis of very early metabolic reactions. ‘In fact, ADP is converted into ATP by receiving energy from the thioester in modern organisms,’ says Matsuo. ‘These facts suggest that thioesters may have been the energy currency of primitive metabolism.’
Transport issues
Several groups have also been looking at how RNA molecules might find their way into coacervate protocells and whether their survival and reactions would be enhanced inside. This includes chemist Dora Tang from the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden, Germany. Tang and her collaborators have been trying to increase the concentration of RNA inside coacervates using systems based on a carboxymethyl dextran sodium salt and the polypeptide poly-L-lysine in the presence of simple ribozymes derived from the tobacco ringspot virus. Using a fluorescent probe, she showed the ribozyme continued to function as a catalyst and cleave RNA molecules inside the coacervate.
Tang was also able to increase the uptake of RNA molecules into the coacervate. ‘We found that different lengths of RNA can be selectively retained within the coacervate,’ she explains. They found that longer RNA molecules, with 39 nucleotides, were better retained than shorter ones of 12 nucleotides. ‘In the case of RNA this is probably associated with charge, so the longer the RNA, the more charge.’
We are a long way from mimicking basic cell behaviour
Her work on coacervate protocells has made Tang think about the various models for the early stages of chemical evolution in a different way. Competing with proponents of the RNA world are those who suggest that primitive protein synthesis developed first, before RNA or DNA, in what’s known as the peptide world. ‘We know that you can form coacervates from RNA and peptides. So is there a possibility that we don’t have disparate ideas, that these could have all accumulated within one droplet and started the onset of life?’ she asks.
In December 2021, Tang, with collaborator Dieter Braun from Ludwig Maximilian University Munich in Germany, published new results suggesting how coacervates may have evolved in the sorts of gas bubbles found inside heated rock pores – one of the suggested locations where life may have begun. Using a temperature gradient to recreate the out-of-equilibrium conditions, they found that they could form cocervate droplets from polyanionic and polycationic species, and that they would accumulate at the gas–water interface where they underwent continued fragmentation that prevented the droplets coalescing into a single phase. The non-equilibrium interface conditions allowed coacervates to form with different compositions to those found in the bulk liquid phase, which Tang sees as a clue to the driving force for the early evolution of coacervates.
But most of the attempts at creating protocells that can grow or replicate molecules inside are barely sustainable. ‘Right now, it does seem so hard to have anything that keeps going for a couple of cycles… some sort of human intervention is always needed,’ says Wang. The fatty acid vesicles she has created that seem to divide ultimately get smaller and smaller until they disappear. What is missing is the ability to use fuel from their surroundings – a metabolism. ‘Ultimately, they’re running down, whereas life, of course, has this out-of-equilibrium state. How we [recreate] that is going to be a big challenge,’ says Mann. ‘We are a long way from really mimicking [that] sort of basic cell behaviour.’ Tang agrees. ‘If you’re building something from scratch, then you don’t have that complexity that allows you to actually sustain the out of equilibrium behaviour. So this is kind of the engineering challenge that we have.’
The problem in building protocells is not the individual components but understanding how they assemble. ‘Even if you have all the components how can they act cooperatively, in an integrated fashion or a systems fashion, to generate what you might consider the rudimentary representation of life?’ asks Mann. ‘It’s not obvious – you could have all the components, just as you could have all the bits and pieces of a computer but if they’re not integrated correctly, and powered, then they will just sit there as pieces of silicon.’
Mann also suggest that perhaps protocells actually developed as groups or communities of cells with slightly different properties, rather than one single prototype. ‘We think of protocells in terms of population dynamics, not individuals so much, but as how they work as a collective.’ Microorganisms today form biofilms where cells exist together within a slimy extracellular matrix. ‘It may well be that the first kind of rudimentary life-like objects were actually aggregates, and therefore a kind of division of labour can take place within them.’
Burden of proof
Whether it will ever be possible to prove the exact mechanism by which cellular life came about four billion years ago is clearly debatable. Wang says most of the origin-of-life research community are just hoping for a way to ‘connect the dots’ of each increasingly more complex step. ‘Then I think some people would say “OK, I’m satisfied, we found one way to get there” – [though] not everyone.’
Mann, who is sceptical that we can ever know what was essentially a historic event, is more interested in the general scientific questions protocell research brings up and the tantalising possibility of discovering if there is life beyond biology – a ‘life 2.0’ that might help us understand the transition between non-living and living matter. ‘In my opinion, that’s much more realistic, although I don’t think we’ll answer [it] in the next 50 years.’ He suspects the key is down to information flow. ‘You could argue that the difference between non-living and living is that information has the upper hand in a living system… information is in the executive position, it’s somehow controlling the matter [of] which it’s made.’
I don’t think we’re going to have protocells climbing out of the test tube
Those working on protocells to discover how life began are limited to scenarios that are biologically plausible, using the molecules we know are found in biology today, but Mann’s search for life 2.0 has allowed him to be a bit more flexible in his design criteria and find applications for these systems. ‘I don’t think we’re saying that we’re going to have protocells climbing out of the test tube, moving along the desk, but we can establish behaviours in these artificial systems, which are representative of the [properties of life], like growth, division, and motility.’
Mann has designed synthetic protocells that can chemically communicate with living cells and tissue, in one case being able to release nitric oxide, a vasodilator. He has created bio-compatible proteinosomes using haemoglobin-containing lipid membrane fragments that are able to circulate in the blood stream. These encapsulate polysaccharide–polynucleotide coacervates, also containing the enzyme glucose oxidase. In the presence of glucose and hydroxyurea, the enzyme catalyses a cascade of reactions leading to the release of nitric oxide, which causes the blood vessels to dilate. The system was tested on rabbits by injecting the protocells and hydroxyrea into their carotid artery. Making use of the animals’ own blood glucose, they were able to produce sufficient levels of nitric oxide to induce an increase in blood vessel circumference in the animals.
For chemists like Matsuo, they hope creating protocells will start to answer the fundamental question of what really divides the living from the non-living, ‘I have been fascinated by these mysteries since I was a middle school student,’ he says. To understand how the first protocells turned into life, Qaio thinks chemists are going to need a lot of help from other disciplines, including the geologists who study the early earth. But ultimately, she admits, understanding life might not be a scientific question at all but one for philosophers.
Rachel Brazil is a science writer based in London, UK











No comments yet