From solar cells and LEDs to catalysts and quantum computing, James Mitchell Crow asks if there is anything perovskites can’t do
Even if you have only glanced casually at a science journal or two over the past few years, you can’t help but to have spotted a trend: a spectacular proliferation of research papers focussed on perovskites.
Perovskite-based photovoltaic (PV) materials in particular have been grabbing headlines for their spectacular recent performance gains at converting sunlight into electricity. But PV is far from the only branch of perovskite research currently enjoying some time in the sun. From catalysis chemistry to light-emitting diode (LED) research, and many places besides, perovskites are the materials of the moment.
‘This is the beauty of perovskites – they have some intrinsic properties that it seems whatever you do, they are somehow excellent,’ says Gabriele Rainò, an optoelectrics researcher in Maksym Kovalenko’s lab at the Swiss Federal Institute of Technology in Zurich. ‘They are good for everything, that is what we have experienced,’ he says.
Putting aside their high profile potential as low cost solar materials, what is behind perovskites’ surging popularity in these diverse research fields, and where are we likely to soon see real world applications?
Catalysing the future
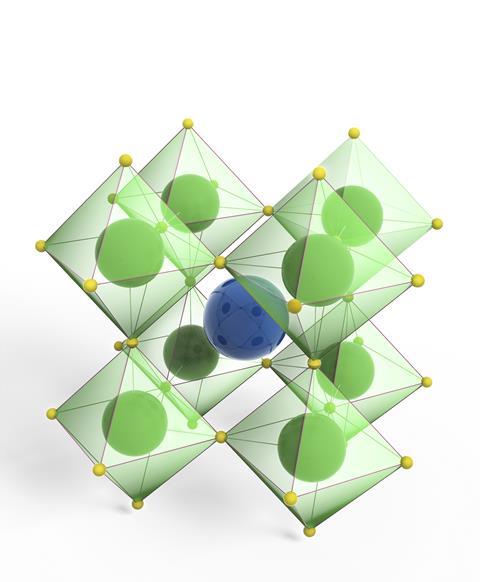
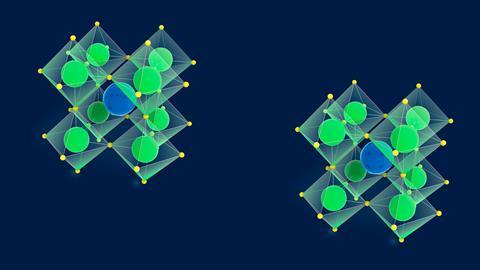
To some extent, perovskites’ all-pervading nature can be attributed to the perovskite family’s sheer size. Perovskites are crystalline materials with the general formula ABX3, where A is a larger cation, B is a smaller cation and X is the anion. The B and X ions are arranged in a cubic structure around each A cation.
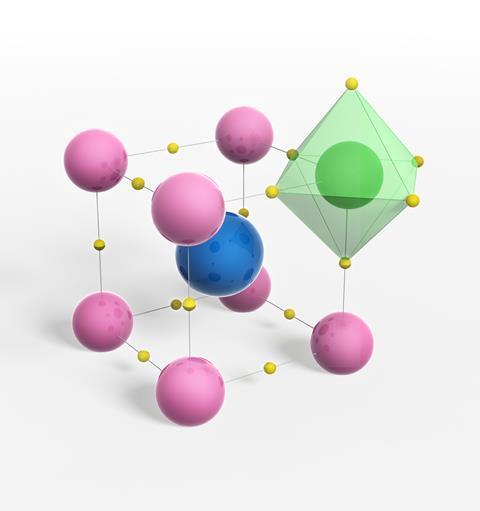
The first perovskites known to science were naturally occurring minerals. Calcium titanate (CaTiO3) was the first member of the family to be unearthed, by German chemist Gustav Rose on an expedition in the Ural mountains in 1839. Rose named the mineral ‘perovskite’, after Russian nobleman and mineralogist Lev Perovski. All subsequently discovered or synthesised materials with the same crystal structure also now carry Perovski’s name.
The perovskite family is so large because there is an incredible flexibility in terms of the ions that can be used to make up each component of the structure. ‘More than 90% of metallic elements in the periodic table can be used in the perovskite structure,’ says Hamidreza Arandiyan, who researches perovskite materials at the University of Sydney in Australia.
Throughout their history, perovskite oxides – in which oxygen filled the anion position and various metals filled position A and B – have always received significant attention. In a review published in Science in 1977, researchers from Bell Laboratories noted that the interesting properties of perovskite oxides ranged from ferroelectricity and piezoelectricity to high-temperature superconductivity – but that surprisingly little work had been done on their potential as catalysts.1 At the time the review was published, the authors noted perovskite oxides’ potential for vehicle catalytic converters, to reduce nitrogen oxides (NOx) emissions to clean up the exhaust gases that were leading to smog. Lead-based perovskites were particularly of interest, because they were impervious to poisoning by the lead added to the petrol of the day.
One idea at that time, which resonates strongly today, was to incorporate small amounts of highly catalytically active precious metals into the perovskite structure. Replacing around 5% of the B cations in the perovskite structure with ruthenium provided an active catalyst while only using a small amount of expensive precious metal. Embedding the ruthenium into the perovskite structure also reduced ‘sintering’, the gradual agglomeration – and so loss of surface area and catalytic activity – of precious metal particles that can afflict catalysts.
One reason perovskites are such a useful and interesting system is that you can get a very diverse chemical set with the same structure
Ultimately, platinum-based catalytic converters won the day, after lead was removed from petrol. But today, perovskite-oxide-based catalysts are of great research interest again, for their potential to clean up the current exhaust gases of environmental concern.
Depending on the metals used, the perovskite’s electronic structure and surface binding energies can be tuned toward oxidation of toxic gases such as carbon monoxide, NOx and certain hydrocarbons; reduction of carbon dioxide with the idea of recycling the greenhouse gas back to useful hydrocarbons; and the oxygen evolution reaction of electrochemical water splitting, a method for turning water into hydrogen fuel.2
For the latter reaction, the current ‘gold standard’ catalysts for oxygen evolution are iridium and ruthenium oxides, which are expensive, says Jonathan Hwang, a PhD student in the lab of Yang Shao-Horn at the Massachusetts Institute of Technology in the US. Perovskites have shown great potential as alternatives without precious metals, he says.
‘One reason perovskites are such a useful and interesting system is that you can get a very diverse chemical set with the same structure,’ Hwang says. ‘You can systematically look at different chemistries from the same structure, and derive some sort of chemical intuition from which to design better catalysts.’ By establishing patterns of reactivity, the rational design of better catalysts becomes possible.
Oxygen evolution catalysis is one area the rational approach is already paying off, Hwang says. Shao-Horn’s lab discovered that strontium cobaltite perovskites had great potential as oxygen evolution catalysts. The pattern of reactivity the team observe has been picked up and extended by other researchers, including a team in China.3 ‘With the rational design parameter, this lab has found a couple of perovskites that are higher activity than ruthenium oxides in basic solution,’ he says.
Reactivity insights gained by systematically changing the perovskites’ chemical make-up are not just limited to perovskites themselves, but can also be applied to catalyst design in general, Hwang says. ‘These systematic studies play well with trends in machine learning and big data science,’ Hwang adds. ‘Perovskites provide a nice framework for doing these kinds of combinatorial searches to discovering new materials.’
At the University of Sydney, Arandiyan is also exploring the catalysis chemistry of perovskites and beyond. He is focused on porous metal oxide materials for energy-related applications. Making perovskites porous maximises the surface area available for catalysis and enhances their performance. Porous perovskite oxides, and related metal oxide materials, can also make excellent supports into which small amounts of noble metals can be incorporated – extending the original idea from the 1970s.
Arandiyan recently made a porous perovskite containing rhodium–nickel nanoparticles that was an active, stable catalyst for reacting hydrogen with carbon dioxide to make methane.4 But he has his sights set on something smaller that nanoparticles. To get maximum catalytic performance from minimal precious metal, the trend is to downsize: from nanoparticles to clusters, and from clusters to two or three atoms, Arandiyan says. ‘Our idea is to work with the single atom.’
Although he hasn’t applied it to perovskite oxides yet, Arandiyan and his team, along with collaborators at the University of New South Wales in Sydney, have successfully tested the idea with simpler metal oxides. The team recently created a cerium oxide catalyst that incorporated single platinum atoms in the surface.5 The catalyst was 7.2 times more active for carbon dioxide reduction than an equivalent material bearing platinum nanoclusters, despite its 40 times lower platinum loading. And the metal oxide is not just there for support: carbon dioxide activation occurs on the metal oxide, the team showed, while hydrogen dissociation occurs on the platinum. Anchoring individual atoms at separate sites on the catalyst surface should ensure long-term high performance by preventing noble metal agglomeration and sintering.
The many faces of perovskites
Batteries
Perovskites aren’t just good for generating solar electricity; they can store it too. Perovskite materials are being investigated as battery electrodes with a high lithium ion storage capacity; as a lithium-ion-conducting solid electrolyte for solid state batteries; and as a ‘photobattery’ that can generate and store electric charge from solar energy.
Magnets
By incorporating different metals into the perovskite crystal structure, all manner of magnetic properties can be incorporated. Researchers recently showed that copper–iron layered perovskites possessed, even at room temperature and above, an exotic form of magnetism called spiral magnetism. The property could be useful in spintronic and other devices
Superconductors
When the first high temperature superconductor was discovered in 1986, it was considered such a milestone that its discoverers, Alex Müller and Georg Bednorz from IBM Research, were awarded the physics Nobel prize just a year later. The copper oxides they were working on were perovskites. Superconductivity seems to be one area where perovskite research is not in the ascendancy; today, hydride materials are the hot topic of high temperature superconductivity research.
Gamma ray detectors
Perovskites absorb more than just the visible portion of the electromagnetic spectrum. Some perovskites can also absorb gamma rays. Current commercially available gamma ray detecting materials are very expensive. The perovskite caesium lead bromide is a low-cost alternative that performs just as well or better, recent research has shown.
Lasers
The light emitting properties of semiconducting perovskites are not limited to LEDs. They can also act as lasers. One possible application is for lead halide perovskite nanolasers, which have potential as on-chip light emitting components for optical computers.
Ferroelectric materials
Several inorganic perovskites, as well as the recently discovered all-organic perovskites, exhibit ferroelectricity: they exhibit spontaneous electric polarization, which can be switched by applying an electric field. The polarization remains when the electric field is removed, which makes these materials of interest as data storage devices, as well as sensors and capacitors.
Hybrid solutions
Chemists with an eye on catalysis may be exploring beyond the classic perovskite structure into other metal oxides. But within the ABX3 formula, there is a lot more that can be done besides playing with different combinations of metal ions in the A and B positions.
A major turning point in the development of perovskite materials was the discovery they were not limited to being purely inorganic materials. Organic molecules, in cationic form, could be incorporated into the ABX3 structure. In the late 1970s, the hybrid organic–inorganic lead halide peroxides were discovered, in which methylammonium (CH3NH3+) or formamidinium (CH(NH2)2+) ions form the B cation of the structure.6 In the late 2000s, hybrid lead halide perovskites were shown to have photovoltaic properties, sparking the rapid rise of perovskite solar cell research (see The power of perovksites).
The interplay between electrons and photons that takes place in hybrid perovskites has potential applications far beyond solar cells. The biggest in the short term is their potential as LEDs for lighting and electronic displays, says Rainò. The same key attributes that make perovskites so appealing as photovoltaic materials – their low-cost fabrication, their defect tolerance and their efficiency at converting light into electricity (or vice versa) – apply to LEDs.
‘These materials are solution processible at room temperature, so we share with PV the benefit that we have a semiconductor that is produced at low cost,’ Rainò says. ‘You can imagine spin-coating over large areas for displays, at relatively low cost.’
Beautiful imperfections
The key to LED (and PV) perovskite performance is that despite this humble fabrication approach, and the inevitable crystal structure defects that result, hybrid organic–inorganic lead halide perovskite semiconductors perform flawlessly. Usually, defects in a semiconductor will act as energy traps that limit luminescence. ‘With perovskites, somehow you can get very bright materials despite their many defects – somehow they behave like a high-quality pure crystalline semiconductor,’ Rainò says.
‘It is a surprise, and I don’t think we have the answer yet,’ agrees Felix Deschler, a perovskite optoelectronics researcher at the University of Cambridge in the UK, who was one of the first researchers to look the photophysics behind the unexpected efficiency of organometal lead halide perovskites as a postdoc in the lab of Richard Friend.7 One possible explanation is that the defects are at unusually high energy, outside of the bandgap, and so don’t act as a trap that impairs the optical properties.
With perovskites, somehow you can get very bright materials despite their many defects
On the one hand, it was a lucky discovery that lead halide hybrid perovskites possess this behaviour. The downside is that using lead in LEDs or solar cells is not ideal, given its toxicity. ‘Trying to find other materials with the same property is actually pretty difficult. Aside from lead halides, all the others are less efficient, so it is unfortunate that lead does the magic,’ Deschler says.
The lead limitation aside, the perovskites’ other great appeal is their incredible chemical flexibility. As with catalysis, the ability to easily and systematically change the perovskites’ component ions is a great part of the appeal of perovskite research. ‘You can change a lot of things but still make a nice material,’ Deschler says. ‘To do this with an organic semiconductor would involve a lot of synthesis that might take one or two years in a chemistry lab.’ With perovskites, it might only take a month.
‘The fact you can easily tune the composition gives you a very high freedom in tunability for light emitting devices,’ Rainò agrees. Switching anion from bromide to chloride to iodide gives light emission that covers all of the visible spectrum. ‘There are barely any material systems where you can do that.’
The quantum factor
As with catalysis, things get interesting when you shrink down to the nanometre domain, Rainò adds. Make them small enough, and lead halide perovskite nanocrystals become single photon emitters, which qualifies them as a quantum light source.
Quantum communications, in which sensitive information is securely transmitted by encoding it into the quantum properties of the photon, is one potential application of perovskite nanocrystals. ‘Since you cannot read these quantum properties without destroying the information, this channel is secure by the law of physics,’ Rainò says. But if the team can also find a way to produce high numbers of entangled single photons, the other possible application is photonic quantum computing.
The promise of photonic quantum computing is that you could build a quantum computer using conventional, classical optical components such as beam splitters. The quantum behaviour comes from using single photons in an entangled quantum state. ‘There are some tricks you can play – if entangled photons reach a beam splitter, then by some quantum mechanical property the two photons will exit the beam splitter on the same path,’ Rainò explains. ‘You can use this to make logic operations.’ The research challenge is to find materials that can rapidly generate high numbers of entangled photons. Devices developed so far generate entangled photons at an agonisingly slow rate – but lead halide hybrid perovskites could change that.
Rainò, Kovalenko and their colleagues recently demonstrated an ensemble of perovskite nanocrystals that self-organise into superlattices of quantum dots.8 This ensemble emitted single photons in a highly correlated state. ‘We didn’t demonstrate entanglement, but they are highly correlated which is the first step in that direction,’ Rainò says.
Lead halide hybrid perovskites’ defect tolerance is again a huge plus for single photon production. If the light emission of a single photon emitter gets quenched by a defect, you get no light emitted at all. For more conventional single photon emitters such as cadmium selenide-based quantum dots, ‘you have to work very hard’ to remove the defects, Rainò says. ‘When I saw this defect tolerance [of perovskites] I thought “Wow, these particles on the single particle level should be beautiful.” Indeed they are.’
All organic
Some 180 years after perovskites were first discovered, new forms are still being found. In 2018, a team in China created perovskites that are entirely metal free, with organic cations filling the A and B parts of the structure and a halide as the anion.9
The discovery opens up a huge new branch of the perovskite family for exploration, says YuMeng You from Southeast University in Nanjing, China, who co-led the work. ‘If we look at the periodic table, at the metals suitable to form the perovskite structure, there are not so many,’ he says. ‘But if you look at organic molecules, there are so many variations. All-organic perovskites give us the flexibility to design the material from very basic elements.’
You and his colleagues created their all-organic perovskites in a bid to make better ferroelectric and piezoelectric materials. Piezoelectric materials generate electricity when placed under strain, or vice versa – a property that has proven useful from sonar and ultrasound imaging to vehicle airbag sensors and computer components. One of the materials that currently dominates the piezoelectric material industry, BaTiO3 (BTO), is an all-inorganic perovskite oxide.
We are not organic chemists – if more organic chemists join this field there will be plenty of variation
BTO represent one of the most significant current real-world applications of perovskite materials. ‘These materials have very good ferroelectric and piezoelectric properties,’ says You. As ceramics, they are relatively inexpensive to produce because they can be formulated as powders, he adds. But because it is a ceramic, BTO is not suitable for flexible thin film applications. ‘These materials are so rigid it is not so easy to bend them to form a flexible structure, and it is not easy to make a very thin 1nm film,’ You says.
Flexible piezoelectrics do exist, in the form of ferroelectric polymers, but their piezoelectric performance is not as good as BTO. All-organic perovskites could sit in the sweet spot, combining flexibility with crystallinity for strong piezoelectric performance, the team suspected. ‘We are trying to combine the advantages of both,’ You says.
And that is exactly what they achieved. MDABCO (N-methyl-N’-diazabicyclo[2.2.2]octonium)–ammonium triiodide is a metal-free perovskite with piezoelectric performance comparable to BTO. ‘Ceramics are heavy and rigid. If we can change to an organic perovskite, devices can be lighter, thinner, smaller, more flexible,’ You says. Applications could include heartbeat sensors in wearable devices, touch-sensing artificial skin for robotics or prosthetics, and lightweight active vibration-damping systems.
Piezoelectric materials are just the start for the all-organic perovskite field, You adds. ‘We are still playing with these all organic perovskites, we still have several properties we are trying to imbue like luminescence and magnetism,’ he says. Using chiral organic molecules in the perovskite structure could generate right- and left-handed materials that interact differently with polarised light, which could have quantum communication applications.
‘One of the good things is that all-organic perovskites are a very big family of materials,’ You says. ‘We are not organic chemists – if more organic chemists join this field there will be plenty of variation,’ he says. ‘It is a huge playground.’
Which succinctly sums up perovskites as a whole.
James Mitchell Crow is a science writer based in Melbourne, Australia
References
1 R J H Voorhoeve et al, Science, 1977, 195, 827 (DOI: 10.1126/science.195.4281.827)
2 J Hwang et al, Science, 2017, 358, 751 (DOI: 10.1126/science.aam7092)
3 S Zhou et al, Nat. Commun., 2016, 7, 11510 (DOI: 10.1038/ncomms11510)
4 H Arandiyan et al, ACS Appl. Mater. Interfaces, 2018, 10, 16352 (DOI: 10.1021/acsami.8b00889)
5 Y Wang et al, ACS Appl. Energy Mater., 2018, 1, 6781 (DOI: 10.1021/acsaem.8b00817)
6 M V Kovalenko, L Protesescu and M I Bodnarchuk, Science, 2017, 358, 745 (DOI: 10.1126/science.aam7093)
7 Z-K Tan et al, Nat. Nanotechnol., 2014, 9, 687 (DOI: 10.1038/nnano.2014.149)
8 G Rainò et al, Nature, 2018, 563, 671 (DOI: 10.1038/s41586-018-0683-0)
9 H-Y Ye et al, Science, 2018, 361, 151 (DOI: 10.1126/science.aas9330)












No comments yet