Nina Notman takes a look at the recent and upcoming diagnostic and screening innovations aiming to drive down the incidence of tuberculosis globally
I was three when I first tested positive for tuberculosis. My mother had recently been diagnosed with TB and was extremely ill in hospital near our home in Essex, UK. The rest of my family had been tested to see if we had TB too. Our story had a happy ending: I was the only additional family member to test positive and both my mother and I recovered fully – after taking antibiotics for nine months.
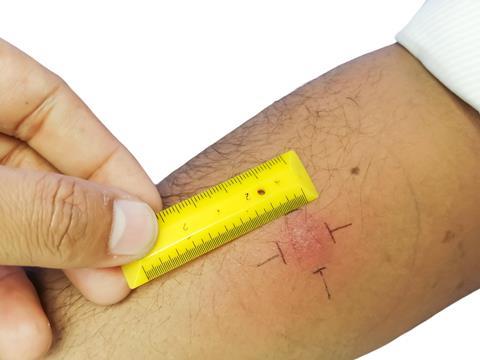
My family’s ordeal was mostly forgotten about until I was 13. As was routine at the time, a school nurse gave me a Heaf test a week before planned Bacille Calmette–Guérin (BCG) vaccinations for my year group. My mother had been unsuccessful at withdrawing me from this process. A Heaf test involves injecting a combination of purified TB proteins called tuberculin just under the skin of the inner forearm with a spring-loaded gun. A circle of bumps over the next few days indicates either current infection or previous exposure to TB bacteria. As expected, I developed bumps and the school nurse team panicked. Two days later, I had an emergency x-ray to satisfy everybody that I wasn’t infectious and therefore not going to cause an outbreak in the school.
Although the Heaf test is rarely used today, another tuberculin skin test called the Mantoux test is still widely used in the UK and around the globe. It was Robert Koch, the German scientist who identified the rod-shaped bacterium Mycobacterium tuberculosis as the cause of TB in 1882, who first reported the skin reaction to tuberculin. He was trying to develop a treatment by killing cultured bacteria and injecting the concoction under the skin. ‘It didn’t turn out to be a cure, but it became useful as a test for TB infection,’ explains Madhukar Pai, associate director of the International TB Centre at McGill University in Montreal, Canada.
Another TB diagnostic originating with Koch is sputum smear microscopy. This remains the most used diagnostic tool for active pulmonary TB today in low and middle income countries. It was how my mother was diagnosed and involves staining a thin smear of sputum – the mucus coughed up from the lungs – with a dye and examining it under a microscope.
Mind the gap
The over-reliance on diagnostic techniques developed over 140 years ago has left TB with a significant diagnostic gap. In 2022, 10.6 million people fell ill with TB, according to the World Health Organization. Fewer than 7.5 million of these people were diagnosed with the disease. This means 3 million people with active TB went undiagnosed – and untreated.
TB can lie latent in the body causing no harm for decades. But active TB that isn’t treated has a 50% mortality rate. The WHO estimates that 1.3 million died from TB in 2022. ‘Tuberculosis is the second leading cause of death from an infectious agent globally,’ says Nazir Ismail, a professor of clinical microbiology and infectious diseases at the University of the Witwatersrand in Johannesburg, South Africa, who until recently worked for the WHO on TB diagnostics policy. TB was the top cause of death from a single infectious agent for decades prior to Covid-19.
There is an UN Sustainable Development Goal to reduce TB incidence by 80% and TB deaths by 90% by 2030. To achieve this goal, better diagnostics will be needed. As Pai and colleagues wrote in Nature Microbiology in May 2023: ‘If we cannot find TB, we cannot treat TB. And if we cannot treat TB, we cannot end TB.’
Molecular diagnostics
The biggest issue with sputum smear microscopy is its lack of sensitivity. ‘Sputum microscopy can barely pick up one of two people with TB disease,’ explains Pai. It works best on samples from people with high bacterial loads in the lungs – and therefore lots of bacteria on the slide .
In recent decades, the WHO has endorsed some more sensitive diagnostic tools. These include molecular diagnostic tools that look for the DNA of M. tuberculosis in sputum samples. Cepheid’s GeneXpert system, endorsed by the WHO in 2010, is the most widely used molecular diagnostic for pulmonary TB. GeneXpert uses cartridges preloaded with reagents. ‘It’s the Nespresso machine of the TB tests,’ Pai says. A consortium of collaborators including the Bill & Melinda Gates Foundation and the Foundation for Innovative New Diagnostics (FIND) supported GeneXpert’s development. The system processes sputum samples in about 90 minutes, extracting, amplifying and detecting DNA from M. tuberculosis.
In the TB world we’ve not had much competition in the diagnostic space
Some of the rapid molecular assays, including GeneXpert, can also identify DNA markers for resistance to rifampicin, a key antibiotic in the treatment of TB. Prior to their use, drug-resistant TB bacteria could only be identified by culturing them in a lab and exposing them to various drugs – a process that can take months. The WHO reports that two-thirds of drug-resistant TB cases go undiagnosed.
In 2010, the WHO started to recommend that rapid molecular assays be used as the initial test to diagnose TB instead of sputum smear microscopy in high-risk groups and, in subsequent years, this recommendation was extended to all individuals thought to have TB. But despite this, the uptake of TB molecular diagnostics has been painfully slow. In 2022, only a third of TB diagnostic sites worldwide had access to molecular tests, according to the WHO.
The reasons are multifaceted but high costs, poor instrument service support in low- and middle-income countries and supply issues are major contributing factors. Cepheid has not met global expectations for GeneXpert. Because of this, a couple of years ago, the WHO started to encourage more companies to develop and manufacture rapid molecular diagnostics for TB. ‘In the TB world we’ve not had much competition in the diagnostic space,’ says Ismail.
New entrants into the market include the Indian company Molbio Diagnostics – the first company based in a low- or middle-income country to gain WHO endorsement for a TB diagnostic. In 2020, the WHO approved the company’s Truenat machine for TB and drug resistant TB testing.
Easier sample types
There is currently significant interest within the TB diagnostics community in developing rapid molecular diagnostics for sample types other than sputum. Between 30 and 40% of the global population who cannot produce sputum samples, including children and people with HIV. By testing additional sample types, it is hoped that the TB diagnostic gap can be reduced.
Tongue swabs are a much simpler matrix than sputum
Tongue swabs are one sample type being explored. The Bill & Melinda Gates Foundation and FIND are part of a large consortium examining their potential for use with rapid molecular tests. ‘We’ve been working to develop a body of evidence that shows that swabbing with the same kind of swabs we used for Covid on the surface of the tongue can be used for TB molecular testing,’ explains Karen Heichman, the deputy director of diagnostics of the Bill & Melinda Gates Foundation in Seattle, US. ‘Looking at the pilot studies and evidence that exists right now, [tongue swabs] look very promising,’ adds Mikashmi Kohli, senior manager of evidence and policy at FIND in Geneva, Switzerland.
Tongue swabs also offer logical advantages. ‘Tongue swabs are a much simpler matrix than sputum,’ says Adithya Cattamanchi, a professor of medicine at the University of California, San Francisco Center for Tuberculosis. As a result, specimen processing is simpler, allowing cheaper, more portable instruments using cheaper consumables to be used.
A clinical trial planned for early 2024 will allow the consortium to gather more evidence on the suitability of tongue swabs for TB diagnostics. The goal is to collect the data needed to file for regulatory approval for the first generation tongue swab system by the end of the year, says Heichman. ‘In two years time, I think we’ll have a few different options [endorsed by the WHO],’ she adds.
Speeding up centralised testing
Tongue swabs are also being explored for use in centralised TB testing laboratories with LGC’s ultrahigh throughput Nexar instrument. This instrument carries out polymerase chain reactions (PCR) on a continuous tape of arrays with 384 wells in each. A single instrument can perform over 100,000 tests per day. The technology was originally developed for the agricultural industry and has been used in the UK for diagnosing Covid-19.
The Bill & Melinda Gates Foundation is currently supporting the validation of the use of tongue swabs and the Nexar technology for scaling up in South Africa’s centralised testing abilities. South Africa has one of the highest TB incidences in the world and already annually tests the sputum of 2.3 million of its 60 million inhabitants for TB. In order to drive down disease levels, even more people need to be tested each year, explains Lesley Scott from the Diagnostic Innovation Hub at the University of the Witwatersrand.
Scott is a member of the team validating the use of Nexar instruments and tongue swabs for diagnosing TB. ‘We envisage a throughput of between 3000 to 5000 [TB] tests per day on a single platform of this type,’ says Scott. In this scenario, tongue swabs would be collected at home or at community clinics and transported to a centralised lab. The turnaround time from the lab receiving a specimen to reporting a result digitally would be around 24 hours, she says.
Urine testing
Urine is another sample type that could help reduce the TB diagnostic gap. In 2015, the WHO endorsed the first, and so far only, urine test for TB: Abbott’s Determine TB LAM Ag test. This lateral flow urine lipoarabinomannan (LAM) assay is a low-cost point-of-care diagnostic specifically designed to detect active TB in people with HIV.
The urine test is like a full body biopsy
‘LAM is a large complex [sugar] molecule that is a component of the TB cell wall,’ explains Grant Theron, a TB diagnostics expert at Stellenbosch University in South Africa. The lateral flow tests have polyclonal anti-LAM antibodies that capture LAM shed into urine by the body. The tests cost about $3 each, take less than 30 minutes, and are very similar to those used for diagnosing Covid-19 and pregnancy. Abbott’s test was developed for diagnosing people with HIV who are severely ill with TB.
Urine tests are now being explored as a cheap and rapid tool for diagnosing TB in severely ill people without HIV too. A significant advantage of urine tests over those for sputum and tongue swabs is that they can diagnose extrapulmonary TB in addition to pulmonary TB. This is important because around 15% of people with active TB have the disease in organs other than the lungs. ‘The urine test is like a full body biopsy,’ says Theron.
LAM-based diagnostics are particularly suited for people with HIV because their kidneys tend to be less efficient at filtering the molecule out, meaning there is a higher concentration of LAM in the urine. The next-generation LAM assays are being designed to be more sensitive and therefore able to detect lower concentrations of LAM – making them suitable for people without HIV. ‘There has been work to develop higher affinity antibodies to better concentrate the antigen within the urine specimen, and to develop readers and other technology that can better detect whether or not the antigen is present than the human eye, which is what lateral flow assays currently rely on,’ says Cattamanchi.
Companies developing next-generation TB urine tests include the Japanese company Fujifilm and Biopromic in Sweden. Clinical trials are expected to begin in early 2024, with that aim of collecting enough data for the WHO and other policy makers to make recommendations by the end of the year, Theron says.
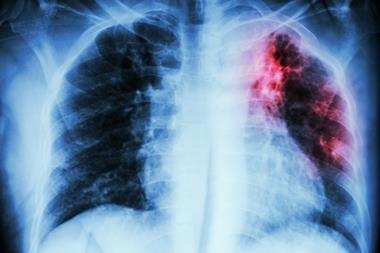
AI supported x-ray screening
Chest x-rays have been used to support the diagnosis of pulmonary TB for over a century and they are still widely used today especially to screen high-risk populations. But because x-rays aren’t specific, they can’t be used alone to diagnose TB. A radiographer can’t easily tell if an observed patch on a lung is caused by TB, lung cancer, pneumonia, a fungus or something else. ‘This is why an abnormal x-ray should always be followed up with the next test, usually a microbiological test or a molecular test,’ says Pai.
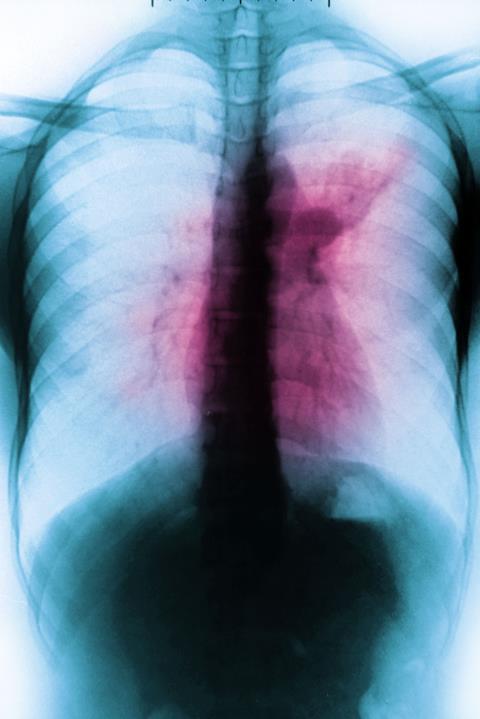
Historically, a lack of radiologists trained to read chest x-rays has limited their use for TB. In 2021, the WHO recommended the use of computer-aided detection (CAD) algorithms to support x-ray reading for TB screening. The algorithms are trained to recognise TB-related abnormalities in the images. ‘There’s been a lot of uptake of computer-aided detection software and the hope is that this will improve the reliability of TB screening where chest x-ray platforms are available,’ Cattamanchi says.
The CAD software processing time is less than a minute and its use has transformed x-ray into a point-of-care screening tool, explains Shibu Vijayan, medical director for global health practice at Qure.ai in Mumbai, India. ‘Previously it took days to weeks to get the x-ray report, then someone has to chase down the patient in the field to get a sample [for sputum testing],’ he adds. Now, it is known whether a sputum sample is needed before the person leaves the clinic.
CAD has also been paired with handheld x-ray machines – meaning screening can take place in the community. ‘The handheld, highly portable systems are quite remarkable. You can directly connect with your laptop, and have artificial intelligence algorithms read the images,’ says Pai. Software from Qure.ai is already being used for TB screening with a number of portable x-ray systems including those manufactured by Fujifilm and Mylab Discovery Solutions in Maharashtra, India.
Breathing out clues
Artificial intelligence and data science approaches are also starting to be explored to analyze sounds that are indicative of pulmonary TB. A cough sound can be recorded with a smartphone and analysed using machine learning algorithms to identify features that are predictive of TB, explains Cattamanchi. His research group, meanwhile, is developing algorithms that can recognise noises representative of TB in lung sounds recorded with a digital stethoscope. ‘Those recordings again become analysable to extract features that are predictive of disease,’ Cattamanchi says. ‘These would be very low cost [diagnostic] tools, if they turned out to be successful.’
Early stage research is also underway to see if exhaled breath is a suitable sample type for diagnosing TB. There are two distinct options being explored. The first is the possibility of detecting volatile organic compound signatures of pulmonary TB in the breath. ‘A couple of universities in South Africa are evaluating this idea in early stage pilot studies,’ explains Kohli.
The detection of M. tuberculosis DNA in the breath is the second possibility starting to be explored. Mike Barer, a professor of clinical microbiology at the University of Leicester, UK, has developed a facemask-based sample collection system for his group’s fundamental research that may also be useful for diagnostics. ‘We use 3D printed polyvinyl alcohol as the sampling matrix,’ he explains. The strips are placed inside a face mask where the breath causes the PVA to become wet so the bacteria can adhere to it. Barer’s lab uses the GeneXpert system to analyse DNA of the trapped bacteria.
The current speed burst of innovation in TB diagnostics has been made possible by the many diagnostic advances seen during the first few years of the Covid-19 pandemic, explains Heichman. ‘Given all of the innovation and the manufacturing and awareness around diagnostics that came with Covid, we are seeking to apply those same principles to TB diagnostic testing,’ she says. But it is simplistic to blame the world’s reliance on TB diagnostic tools developed over a century ago entirely on a lack of prior innovation in this area. Theron explains that high prices and logistical issues for newer diagnostic tools coupled with poor policy decisions have significantly contributed to the slow uptake of new testing technologies. The Covid-19 pandemic shows that point-of-care tests can be rolled out at scale in low- or middle-income countries when there is a political drive to do so, he adds.
Nina Notman is a science writer based in Salisbury, UK














No comments yet