The Getty Conservation Institute in Los Angeles is developing innovative new approaches to art conservation. Fiona Case paid them a visit
This is an exciting and critical time in conservation science. ‘New techniques are revolutionising our ability to characterise the materials used by artists across the ages. We are reassessing long-established conservation protocols and facing new challenges as materials used by 20th century artists enter collections and start to age,’ says Tom Learner, the head of the science department at the Getty Conservation Institute (GCI) in Los Angeles, US. Researchers at the GCI are even questioning the tight environmental control routinely used at art museums.
During the second world war, Germany’s Nazi government stockpiled over 6500 pieces of looted art – destined for a future Führermuseum – in the Salzkammergut salt mine in Austria. After the war it was feared that the pieces, including works by Michelangelo and Vermeer, had been irreparably damaged. But when the paintings were retrieved quite the opposite was true. They had kept beautifully in the dark humidity and constant temperatures of the mine. This informed conservation practices in the postwar years.
‘Art museums and private collectors around the world installed complex air-handing units to maintain 45–55% relative humidity with a temperature between 70–75°F (21–24°C). But is this tight control really necessary?’ asks Learner. ‘If we could relax the conditions by 10%, it would make museums much more sustainable.’
How to preserve a masterpiece
Researchers at the GCI are using nanoindentation microscopy to answer this question. ‘We can use microscopic samples from paintings, or we can create mockups of paintings using historically accurate materials and artificially age them,’ explains Learner. ‘We then place them in an environmental chamber and measure how the physical properties respond to changes in temperature and humidity.’

In nanoindentation a very small force is applied to a very small area using a tip with a very precisely characterised geometry. The applied load and the resulting displacement into the surface being studied are reported while the indentation is in progress. Since the area of the tip is known, the technique gives a real-time measure of mechanical properties which are reproducible from sample to sample, and from one lab to another. And, critically for the analysis of paintings, the technique can be focused on a precise area.
‘The surface of each layer of paint and the interface between layers is very different to the bulk,’ explains Learner. ‘The team assess the physical characteristics of the paint – has a layer become brittle, or sticky? We look for signs of oxidation or degradation.’
Increasing humidity can plasticise the paint and make it sticky. Lower temperatures can accelerate phase separation and cracking. Mechanical failure at the interfaces can lead to delamination. But these issues are only seen with more extreme changes in environment. ‘People should understand that their objects can withstand some fluctuation in conditions – 90% of most collections probably don’t need such a precise level of environmental control,’ says Learner.
The perfect surface
Lacquer is the poster child for new techniques in conservation research. ‘This is a class of material that has been widely used, but could not be well characterised,’ says Michael Schilling, the head of materials characterisation research at the GCI. ‘New methods are allowing us to set up conservation practices and to distinguish different types of lacquers.’

Lacquer is an enigmatic material, strong and beautiful but sensitive to the aging effects of light. ‘Put a drop of water on aged lacquer and you will see the indentation. A fingerprint stays there – you can’t remove it completely,’ says Schilling.
Conservators around the world face a challenge in maintaining materials that were created by artisans in many different countries, across thousands of years. The key ingredient in lacquer is sap harvested from specific species of Asian trees. The tree sap contains complex polyphenols (specifically catechols) and a laccase enzyme that quickly reacts to bark damage, crosslinking catechols to create an impervious film which protects the tree.
‘Other materials are used in lacquers, beyond the sap, are called drying oils,’ explains Michael Schilling, the head of materials characterisation research at the GCI. ‘Materials from seed oils to wood distillates, fruit juices and blood have been used – wildly different recipes were developed by different artisans at different times, and for different layers within an individual object.’
Cutting with drying oils, particularly in the lower layers, is a cost-effective way to make lacquer, and the right additives can allow the material to flow and create a smoother surface. But the results, the black or red materials we call lacquer, are chemically complex and extremely variable. It is not clear that there is a ‘one size fits all’ solution to cleaning and conservation.
Schilling and his team at the GCI have developed a new technique for characterising lacquer to discover what is actually in a specific layer of a specific material.
‘We treat the lacquer with tetramethylammonium hydroxide (TMAH), a methylation agent, and pyrolyse the entire sample at 550°C,’ he explains. ‘Then we analyse the vapours with GC/MS. The resulting pyrograms have 200 peaks or more, but by comparing to a library of traces developed by analysing reference samples we can identify 1400 marker compounds. These are the fingerprints for even the most unusual additives.’
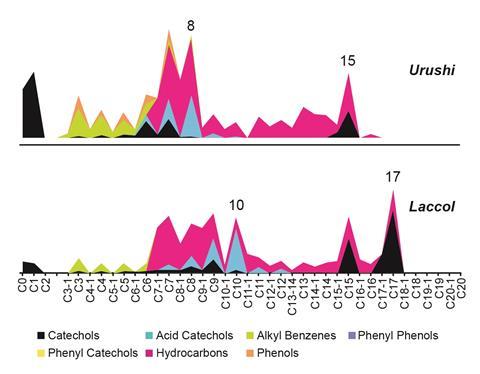
The pyrolysis–gas chromatography/mass spectrometry (Py-GC/MS) technique can reveal the secret, and sometimes strange, recipes of the lacquer makers. Microexcavation of lacquer cross-sections makes it possible to analyse the materials in individual layers. The technique can also distinguish which of the three main species of lacquer trees was the source of sap for a specific object. This is of cultural interest and also important information for conservation. The tree saps have similar proportions of water, plant gum, glycoprotein, laccase enzyme and polyphenols. The main differences lie in the chemical structures of the catechol side groups – and this affects the polymerisation in the lacquer and its flexibility.
The new techniques are being shared with museums and conservation groups around the world through workshops and are also informing conservation work at the Getty Museum. ‘We hope to be able to provide safe cleaning recipes for specific lacquers – to formulate a microemulsion that will cause minimum damage to the aged lacquer, which doesn’t exist now,’ explains Schilling.
The challenge of the modern
With ancient masterpieces, the focus is on maintenance and safe practices for cleaning. ‘History has already filtered those collections – only the most stable pieces remain,’ says Odile Madden, a senior scientist at the GCI. ‘But with modern and contemporary art the challenges are different. Artists are innovators. They often use material at the very edge of their capabilities or introduce new experimental materials.’
‘We have identified a crucial area of research,’ says Learner. ‘Art pieces were made in the 1930s and 1940s using revolutionary materials that were just being discovered – plastics. Many pieces are showing signs of deterioration. The work to understand these early plastics hasn’t been done.’
The instability of the first plastics may not be surprising. The partial nitration of cellulose using a mixture of nitric acid and sulfuric acid (the catalyst) was first developed to produce gun cotton – a more explosive replacement to black power that revolutionised munitions and mining in the mid-19th century. In 1856 the process was modified to produce cellulose nitrate plastic. The addition of the first plasticisers allowed the polymer to be melted and moulded.
The materials lose their transparency, discolour and then disintegrate
Artists such as Naum Gabo and Antoine Pevsner were entranced by this new material and created works that pioneered modern sculpture. The first movies were shot using nitrate film, and the first animated films were painted on celluloid. Some of the most famous artworks and artifacts of the 20th century were created using cellulose nitrate. ‘A lot of it is already gone,’ says Madden. ‘The materials lose their transparency, discolour and then disintegrate.’
The primary decomposition – initiated by UV light or trace amounts of sulfuric acid remaining from the synthesis – is relatively slow. But as the degradation products build up, they catalyse further de-polymerisation. The material falls apart and can, in some cases, auto-ignite.

Cellulose acetate was lauded as a safer replacement and indeed a cellulose acetate object will not spontaneously burn, but it still presents a considerable conservation challenge. ‘I was studying cellulose acetate world war two aircraft recognition models from the National Air and Space Museum – they were showing considerable degradation – when I heard a ping and saw a piece fly upwards. It went a surprising distance across the room,’ says Madden.
You can smell it – “vinegar syndrome” is recognised by conservators around the world
The primary culprit, Madden believes, is the triphenylphosphate plasticiser used in these early cellulose acetate objects. ‘We can track the distribution of the plasticiserwith FT-Raman and x-ray fluorescence spectroscopy. Triphenylphosphate phase-separates and diffuses to the surface. It is a solid at room temperature and crystallises within the objects and on surfaces, in crevices and breakage areas.’
The polymer can also start to degrade. Cellulose acetate breaks down by hydrolysis in the presence of water and releases acetic acid, which then catalyses the cleavage of the 1,4-beta glycosidic linkages in the polymer backbone. ‘You can smell it – “vinegar syndrome” is recognised by conservators around the world,’ says Madden.
Conservators attempt to hold back the polymer degradation by storing cellulose acetate plastics under well-ventilated conditions with materials such as zeolites to absorb the acetic acid vapour, but Madden sounds a caution. ’The competing problem of plasticiser loss is driven by volatilisation at the surface,’ she says. ’Exchanging the air around the object can result in mass loss of as high as 30%. The cellulose acetate shrinks and the remaining material develops stresses. A 3D object that can’t distort to accommodate the stress will shatter.’
Plastic fantastic?
Polyvinyl chloride, PVC, was one of the first fossil-oil based plastics. Many generations may be able to share the exuberant joy that 1960s artists expressed in bright colours, clear films and shiny textures. But there are still conservation challenges, and recent research is sounding a caution on some museum practices. Again the key issue is the diffusion of plasticisers.
‘PVC is often plasticised by diethylhexyl phthalate; this diffuses out of the material over time. It can make the surface of the object sticky. It attracts dust and even sticks to the shelf. It is common to remove this sticky surface – to clean the object – but there are questions about how it affects the ongoing stability of the surface,’ says Madden. ‘Since this is a diffusion-controlled process, this practice could set up a potential for further diffusion, and eventually to the embrittlement of the PVC.’
Preserving the intent
Contemporary art presents another set of challenges for conservators: if the artist is still alive she or he may have an opinion on the preservation of their work.

‘If a Rembrandt painting has cracks conservators wouldn’t repaint a face; we wouldn’t make extensive changes to return that painting to its original state,’ says Learner. ‘For works by artists that are deceased the trend is for conservation to be more conservative. In the recent past, when cracks appeared in Mondrian paintings they were fixed. Now we value his pieces as they are – we value the original paint which the artist used, rather than a work that has been extensively re-touched.’
But the approach is different for contemporary art. If an artist is still alive, the thought behind the art – their intent – may be more important that the material itself. The enormous highly polished polyester sculptures created by De Wain Valentine are a case in point.
‘The 1970’s was an amazingly inventive time for art,’ said Learner. ‘Californian artists were using new plastics and resins – materials that were originally created for the local aerospace industry and car industry – or ideas that were pioneered for surfboard technology.’ Valentine’s work in the 1970’s was truly innovative. He even had to redesign the polyester resin formulations that he used to pour such huge sculptures.
‘He changed the chemistry to slow down the crosslinking reaction otherwise it got too hot. Smoke started to appear from the wooden moulds,’ says Learner. But time passed and the polymer continued to crosslink. In particular, ridges appeared on the surface of Gray Column, a 3m by 2m slab, weighing just under two tonnes, that was intended to be perfectly flat. What should the art conservator do?
‘We could let the piece age,’ says Learner. ‘This is late 1970s plastic, this is what it does. Or we could sand the piece to remove the ripples – which is certainly what Valentine would have done if they had appeared when the piece was first cast; his intent was for a very smooth shiny surface.’
Eventually all objects go back to the earth
But once the decision is made to restore the sculpture, to respect the intent of the artist, there is still a dilemma. ‘Should we use the sandpaper that was available in the 70s?’ ask Learner. ‘Modern sandpaper is capable of providing a far greater level of shine on polyester.’ These types of considerations are important as the conservation field moves on.
‘The artist may want the piece to look pristine for ever – but this may not be possible,’ says Learner. ‘And the artist’s intent can change. When the piece is first made they may say that it’s OK for it to fall apart, but once that the piece is in a collection, has significant value, they may change their opinion.’
Research is active in the field of art conservation. New techniques are being brought to bear, particularly on the challenges of conserving modern and contemporary art from the plastics research at the Getty to conservation work on lobster telephones, sharks in formalin, and furry teacups. Insights from the leading conservation research institutes are fanning out to inform practices in art museums and private collections across the world. There are new strategies for restoration, cleaning, and even for environmental conditions in art museums.
But sometimes it is impossible to conserve a work of art – to preserve that moment of creation and the intent of the artist – for future generations. ‘People want to hear that we can keep these objects forever, but with plastics we can only delay the process,’ says Madden. ‘Eventually all objects go back to the earth.’
Fiona Case is a science writer based in San Diego, US
Article updated 26 June to clarify details about lacquers and the use of PVC.
Further reading
- The Getty Conservation Institute https://bit.ly/2kALuQ6
- M R Schilling et al, Stud. Conserv., 2016, 61, 3 (DOI: 10.1080/00393630.2016.1230978)
- From start to finish: De Wain Valentine’s Gray Column, YouTube https://bit.ly/2J24RMh













No comments yet