They’re not like solid metals or like other liquids, but scientists are starting to understand and exploit them. James Mitchell Crow reports
There’s something compelling, even a little supernatural, about a metal in the liquid state. Liquid metals carry the heft, density and silvery sheen of their solid counterparts, but add a confounding fluidity. They’re unlike other metals, but unlike other liquids either.
Mercury, for example, behaves quite differently to oils or water. A shimmering pool of the metal, when agitated, can break into beads that will skitter across a dish, only to coalesce like a self-healing molten mirror. Elemental mercury can be mesmeric, even at the safe remove of a YouTube video.
Gallium displays a different kind of magic. At typical room temperatures the element is a shiny solid block that might pass for any number of metals. But placed in your palm, gallium will liquify. The low-toxicity metal melts at 29.76°C, a few degrees below body temperature.
‘Liquid metals are extraordinary,’ says Kourosh Kalantar-Zadeh, who studies them at the University of Sydney, Australia. ‘Any time we melt a metal, suddenly it becomes a different creature,’ he says. In the solid state, a metal’s electrons are delocalised, but its ions are locked in place. ‘In a liquid metal, the ions and the electrons move around freely,’ he says. This inherent dynamism is drawing interest in liquid metals for numerous potential applications. Not least catalysis.
Liquid gallium can be spiked with catalytic species to produce alloys with activity far higher than comparable solid catalyst systems. Liquid metals’ fluid nature appears to act as more than just a convenient host system for single atom catalysis. Research increasingly suggests liquid metal catalysts could make waves in industrial and green energy applications.
Through the looking glass
While gallium was discovered in 1875, humanity’s relationship with mercury dates back millennia. Early alchemists knew how to isolate the element from the mineral cinnabar, and believed that mercury was a common constituent of all metals, a sort of metallic essence in liquid form.
Humanity has put mercury to use for a long time. It can be deployed in a simple – if highly hazardous to health – method for extracting gold from ore. More recently, it found widespread use in the electrochemical process for manufacturing chlorine and sodium hydroxide from sodium chloride.
Although mercury’s toxicity has seen its use decline significantly over the past few decades, interest in liquid metal systems is now growing rapidly again, due to the increasing appreciation for gallium.
Despite their superficial similarities as room temperature liquid metals, gallium and mercury are very different chemical beasts, notes Nicola Gaston from the University of Auckland in New Zealand, who uses computational approaches to study liquid metal systems. ‘For one thing, the reasons that mercury and gallium are liquid at room temperature are completely different,’ she says.
Is it individual atoms floating around in there, or do they form complexes or clusters? We had no idea
Filled electron shells, and the effects of relativity as electrons orbit the massive mercury nucleus, result in mercury–mercury bonds so weak the element melts at –38.83°C. With gallium, unusual bonding of a different kind leads to its low barrier to melting. The element – a post-transition p-block metal - features a covalent bond in its structure.
‘Gallium is more like a molecular crystal,’ Gaston says. When a gallium cluster becomes sufficiently small, 100 atoms or less, it morphs from liquid droplet into a two-dimensional structure under the influence of its unusual bonding. ‘This two-dimensional structure is the same, more or less, as the surface structure of liquid gallium,’ Gaston says. ‘So it’s very much a network of atoms, it’s not really individual atoms disconnected from each other as you might think of in a regular liquid.’
Once in liquid form, gallium likes to stay there. Its boiling point is 2400°C, and as a liquid its vapour pressure is virtually zero. Liquid gallium also displays supercooling; it doesn’t refreeze until close to –30°C, almost 60°C below its melting point (see Elemental oddity box below for more on gallium).
Kalantar-Zadeh first met this unusual metal just over a decade ago, when a colleague in the neighbouring office showed him Galinstan, an alloy of gallium, tin and indium that melts at –19°C. ‘I was not familiar with liquid metals, but when I saw Galinstan I was fascinated,’ Kalantar-Zadeh says.
Kalantar-Zadeh’s group was exploring liquid metal properties such as their electrical conductivity and shape-shifting behaviour when Torben Daeneke joined as a postdoc. ‘We wrote a large literature review – and realised that from a chemistry point of view, we had no idea what happens inside liquid metals,’ says Daeneke, who now leads the liquid metal research group at RMIT University in Melbourne, Australia. ‘As chemists, we understand solvation chemistry in molecular solvents, in water, in ionic liquids – it’s been studied to death. But there is this entire other sort of liquid out there, that nobody ever looked at.’
The challenge had been the lack of tools to interrogate a system where you dissolve, say, a small amount of copper in gallium. ‘Is it individual copper atoms floating around in there, do they form complexes or clusters – we had no idea,’ Daeneke says. These shiny metallic systems strongly absorb x-rays, so x-ray analysis is difficult, while light bounces off the reflective surface so UV/vis spectroscopy isn’t effective. NMR isn’t applicable. ‘And we can’t do mass spec because it’s going to ruin the instrument,’ Daeneke says.
New analytical approaches have recently emerged. ‘We are just arriving at a point where we can start to probe these systems using electron microscopy, electron energy loss spectroscopy (EELS), synchrotron and neutron techniques,’ Daeneke says. ‘We’ve been doing a lot of work at the Australian Synchrotron looking at solvation chemistry.’ The contribution of computational chemists to understanding these systems has also been critical, he adds.
‘All of this has just matured, so that’s one of the big drivers of interest for liquid metal research,’ Daeneke says. The second major aspect, he notes, is the demand for more effective industrial catalysts as we confront climate change and the need to decarbonise the economy. ‘Finding better ways to make ammonia, to make hydrogen, to get rid of carbon dioxide – a lot of liquid metals research is moving in that direction.’
Elemental oddity
There is a lot more to the strange case of gallium chemistry than just catalysis. Many recent examples come from the labs of Michael Dickey of North Carolina State University, US.
Dickey first came upon the metal’s unusual behaviour as a postdoc. Colleagues in the lab were exploring the idea of making gallium electrodes, when they noticed the gallium droplets they were depositing didn’t form the expected curved hemisphere shape such as a water droplet would adopt, but instead formed a cone. ‘That’s very strange,’ Dickey says. ‘So, we investigated.’
I can’t imagine a more interesting element in terms of its weird properties
It turned out that, as the gallium was printed, an oxide skin rapidly formed across the metal’s surface that allows it to hold its shape, countering the effects of surface tension that would otherwise pull it into a hemisphere. ‘I think of it like a waterbed, where you’ve got this liquid inside an outer skin,’ Dickey says. ‘It gives gallium really neat properties, which set me down a path of thinking what we can use this for.’
The team showed they could use this oxide skin formation to build complex shapes by 3D printing gallium. ‘It was the first time you can print metals at room temperature,’ he says. ‘If you tried to do this with water, you just end up with a big puddle.’
A decade and a half later, Dickey is still discovering new quirks of gallium behaviour that suggest novel applications. ‘I can’t imagine a more interesting element in terms of its weird properties,’ he says. For example, his group has shown how the flexible, conductive metal can be used in an energy harvesting device, and for stretchy electronic wiring – for example, for headphone wires. ‘As we stretch it, there’s no degradation in sound quality - you get the conductivity of the metal, but the stretchability of a rubber.’
When a gallium droplet is kept away from oxygen to prevent oxide layer formation, the metal has a surface tension 10 times that of water. ‘One of the unexpected things we’ve found was that, if you apply a voltage, you can lower that surface tension significantly,’ Dickey says. One volt applied to a sphere of the pristine metal will cause oxidation at its surface, disrupting surface tension so the droplet starts to spread in a fractal pattern. The moment the voltage is removed, the metal snaps back into droplets.
Dickey has collaborated with industry to make gallium-based shape-shifting antenna for wireless communication, and liquid metal coolants for computer chips that more efficiently ferry away heat than a conventional solid heat sink. Most recently, the team showed that gallium coated onto a polymer could form a long-lived hermetic seal for flexible, stretchable batteries.
‘When I started in the field 15 years ago, I was almost embarrassed to tell people I was working on liquid metals,’ Dickey says. ‘Part of my journey has been trying to convince people why this is interesting. Now, it doesn’t require much convincing because there’s so many people working on it. Catalysis is one of the really interesting things it can do,’ he adds.
Flow state
Over the decades, supported liquid-phase catalysts have regularly been explored for their potential to combine the robustness and ready recovery of heterogeneous catalysis, with the activity and selectivity advantages of homogeneous molecular catalysts. From the early 2000s, for example, supported ionic liquids as hosts for transition metal catalyst complexes were investigated. Ionic liquids’ limited thermal stability restricted their application, however.
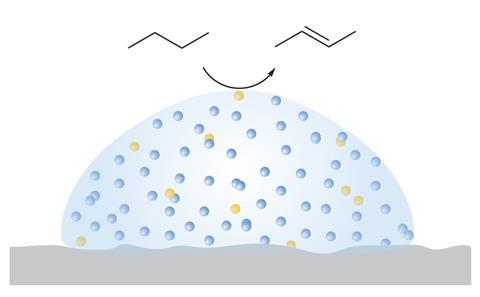
In 2017, a team led by Peter Wasserscheid in Friedrich-Alexander-University in Erlangen, Germany, extended the idea to liquid metals. A handful of studies had shown that solid, palladium-rich palladium–gallium intermetallic compounds were active catalysts for industrial processes such as methanol steam reforming and alkyne partial hydrogenation. Adjusting the ratio of the metals, to produce gallium-rich mixes, should allow access to liquid metal catalysts with far high thermal stability than ionic liquids, the researchers realised. They named the concept supported catalytically active liquid metal solutions (Scalms).
These liquid metal catalysts proved highly effective and notably long-lived, the researchers showed in their initial alkane dehydrogenation study. Organic substrates do not dissolve into the liquid metal but are catalytically converted at its surface. ‘We demonstrate that these supported gallium-rich palladium–gallium phases are liquids under the typical reaction conditions applied for butane dehydrogenation,’ the researchers wrote. ‘Most remarkably, their liquid nature not only provides excellent catalytic activity, but also effectively prevents deactivation, for example, by coking.’
Liquid metal catalysts quickly began to be explored for other reactions. For one thing, liquid gallium-based catalysts can be very simple to make, often just by mechanical mixing. ‘You can put a bit of platinum in, a bit of copper, and make complex catalytic systems very, very easily,’ Daeneke says. ‘There’s also now a lot of evidence that the process of dissolving an element into the liquid metal can really alter the electronic structure of those elements, and make bad catalysts into good catalysts, or good catalyst into excellent catalysts,’ he says. Of course, it can make a good catalyst into a bad one if the wrong components are mixed.
Since the first Scalms systems, liquid metal catalysts’ resistance to deactivation processes is proving to be a key advantage compared to industrial heterogeneous solid catalysts. ‘If you’ve got a liquid surface, processes such as coking and catalyst poisoning basically just can’t occur,’ Daeneke says. Coking is the build-up of carbonaceous residues on the catalyst surface that choke catalyst activity by blocking off active sites. ‘Coke formation on solid versus liquid catalysts is a bit like the difference between trying to stick chewing gum to a wall versus to a bowl of water,’ Daeneke says. ‘The water has no surface the gum can adhere to, it can’t stick.’
The fluid motion of catalytic particles in the system also seems to help them shed potential poisons. ‘If you’ve got a little bit of, let’s say, carbon monoxide in the system, it might absorb onto your catalytic site - but that catalytic site can simply move away, dissolve back into the bulk and pop out somewhere else,’ Daeneke says.
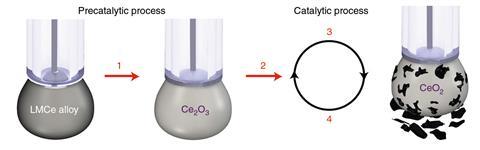
Daeneke and Kalantar-Zadeh teamed up to exploit this anti-coking activity in a catalytic process specifically designed to produce solid carbon deposits. ‘We developed a process to turn carbon dioxide into solid carbon,’ Daeneke says. ‘It’s like un-burning coal.’ They made a gallium-based liquid metal electrocatalyst containing metallic cerium nanoparticles as the active species. This system electrochemically reduced carbon dioxide to layered solid carbon material similar to graphene oxide, which could be harvested from the liquid metal surface.
‘As well as the electrochemical approach, it can be done in a chemical looping approach,’ Daeneke says. The liquid metal itself can react with the carbon dioxide and become oxidised, then regenerated in a second step. Daeneke is working with an industrial partner to scale up the process for carbon-neutral cement. Concrete production cannot be decarbonised because converting limestone into cement forms carbon dioxide as a reaction by-product. ‘We take that carbon dioxide, turn it into graphene oxide flakes, and add these back into the cement and make stronger cement,’ Daeneke says. Other researchers have explored liquid metals for methane pyrolysis, a process that produces hydrogen from natural gas but produces solid carbon, rather than the usual carbon dioxide, as a by-product.
Self-healing systems
Even if liquid metal catalysts do succumb to deactivation, their inherent fluidity can be exploited to induce self-healing, offering an additional advantage for industrial use, says Yingpeng Wu from Hunan University, China.
After exploring gallium-based self-healing battery electrodes, Wu and his colleagues recently extended the work into electrocatalysis. ‘We tried to see if we could find a self-healing catalyst for carbon dioxide electroreduction,’ Wu says.
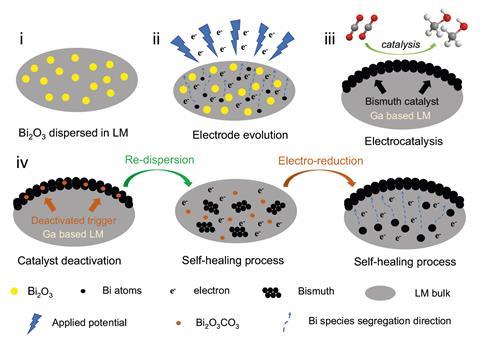
The team mixed bismuth oxide into gallium, then applied a negative potential to generate electrocatalytically active bismuth particles dispersed across the liquid metal surface. This material catalysed carbon dioxide’s electrochemical reduction to formate, until its performance eventually degraded. ‘But by pressing the deactivated bismuth back into the liquid, and re-applying negative potential, the bismuth was regenerated, its structure and catalytic behaviour fully recovered,’ Wu says.
The electrocatalyst could be useful for a range of reductive reactions, Wu says, and the team is now exploring related liquid metal electrodes for oxidation reactions. ‘I think it has great potential for industrial use,’ Wu says. ‘If the electrode can self-heal in situ just by applying a potential, rather than having to be removed and replaced, that will save a lot of time and money.’
Liquid metals might sound exotic for industrial use, but there are a surprising number of precedents, even beyond the mercury electrochemical chloralkali process, Daeneke says. ‘People often think liquid metal is going to be difficult to handle, but it’s already used routinely. The steel industry handles huge amounts of liquid metal every day,’ he says. Nuclear power plants often use liquid metals as coolants. ‘And there’s processes like zinc galvanisation, where you’ve got a big bucket of molten zinc you dip your entire car body into.’
The bigger challenge to scale up might be the cost of gallium, which currently goes for upward of $600 (£470) per kilogram. But there are reasons why that may not always be the case, Daeneke argues. Although gallium is not found in highly concentrated deposits, it is not a rare element in the Earth’s crust. ‘There’s huge amounts of gallium being dug up together with aluminium and zinc, but it doesn’t get extracted because at the moment the only use for gallium is in the semiconductor industry.’ The gallium that is produced is made into an expensive ultra-high purity semiconductor grade. ‘To produce more of it, you could just add an extra extraction stage to an aluminium production facility,’ Daeneke argues. ‘There’s huge potential there to make it.’
Network influencers
Liquid metal catalysts can not only be more robust than their solid counterparts, but far more active. In one striking recent example, Kalantar-Zadeh showed that adding platinum to gallium at atomic concentrations as low as 0.0001% produced a catalyst three orders of magnitude more active than comparable state of the art solid platinum on carbon systems for methanol and pyrogallol oxidation. Pure gallium, in contrast, has no activity for this reaction.
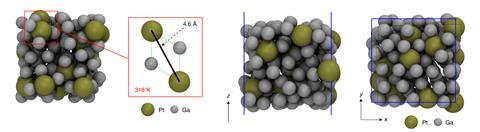
There’s more to the activity of this system than simple platinum single atom catalysis, Kalantar-Zadeh says. ‘The platinum gets encapsulated by gallium atoms, which means the platinum atoms don’t agglomerate,’ Kalantar-Zadeh says. ‘But they also activate the surrounding gallium atoms to become platinum-like, so the surface becomes very active.’
In fact, the platinum atoms appear to have no direct interaction with the reactants at all. Gaston recently completed ab initio molecular dynamics simulations to explore this system in more detail. Understandably, Gaston says, the initial assumption in the literature was that – as in a solid catalyst – the catalytically active metal must be on the surface for the reactant to interact with it. ‘In our simulations, the surprising thing was that the platinum doesn’t actually want to be exposed at the surface,’ Gaston says. Throughout the simulation, platinum never came to the surface, but was continually covered with a layer of gallium.
The high catalytic activity seemed to be due to subsurface platinum atoms changing the electronic structure of gallium atoms around them to become catalytically active. ‘The platinum wasn’t standing up and running the whole show, it’s just sitting there relatively quietly, subtly influencing what was going on around it,’ Gaston says. The unusual network of gallium–gallium bonds across its surface could be key to this behaviour, Gaston says. ‘I think part of why the effects of the platinum might be so strong is that it’s really disrupting a network of electronic behaviour,’ she says.
‘The key question for me is what’s really different about a liquid catalyst compared with a solid catalyst,’ she says. ‘The dynamics of the liquid itself might change the way the catalysis happens.’ Enzymes, for example, are dynamic catalysts that can shape-shift around their substrate to favour certain products.
This more complex viewpoint on the nature of the liquid metal active site, compared to single platinum atoms sitting on the surface, might underpin an understanding for how liquid metal catalysts may be different from solid catalysts, Gaston says. For one thing, the discovery may mean that cheap and abundant metals might be able to replace the platinum or other precious metal in the system. ‘Because it’s not as simple as a single atom catalyst, that definitely might mean different metals can mimic some of the same electronic states for some purposes.’
Fluid future
In Daeneke’s lab, recent work has focused on a liquid metal catalyst version of the Haber–Bosch process for ammonia production. ‘My feeling is that liquid metals are excellent for these reactions where you’ve got gas phase reaction of small molecules, and a lot of issues like catalyst poisoning, coking, deactivation,’ Daeneke says. ‘That’s where a lot of our bulk chemistry resides, making ammonia, hydrogen, polymer feedstocks. Those are the big reactions that we need to learn how to do better.’
When it comes to fine chemical or pharmaceutical production, Daeneke is less certain of their potential. ‘I think catalytic processes where you’re trying to get very selective reactions, I’m not convinced yet that liquid metal can do that – just because the liquid metal surface is amorphous,’ he says. ‘But I’d be happy to be proven wrong.’
Gaston hopes to do so. ‘One of the things that we can imagine happening in liquid catalysts is that the kinetic energy of the metal atoms destabilizes some bonds preferentially, in a way that you wouldn’t have in a solid catalytic environment,’ she says. ‘That inherent dynamics could contribute to selectivity for a particular pathway.
‘The catalyst potential, I think, is massive,’ Gaston adds. ‘On the one hand, there’s the idea that they’re a cheap and simple host environment for precious metals single atom catalysts. And that’s true. But I think there’s a whole lot more to it from the dynamic nature of catalysts that we should be able to unpack and put to good use.’
James Mitchell Crow is a science writer based in Melbourne, Australia

















No comments yet