Kathryn Harkup explores the poisons – real and fictional – used in Bond films
The latest James Bond film, No Time to Die, has been postponed twice already and is now due for release in September 2021. The anticipation, as always, has been very high. Every new 007 film has to live up to, if not exceed, its predecessors. Explosions get bigger, stunts get more intricate and plots often get more ridiculous, only to be scaled back to basics and the cycle starts again. To satisfy the audience’s craving for fast-paced entertainment, details such as credibility and accuracy sometimes need to be sacrificed. But this is far from a complaint: I’m not expecting a chemistry lecture. My expectations of a Bond film are thrilling action sequences, narrow escapes and sinister supervillains, which they deliver in spades.
The films, and the books for that matter, may not be known for their water-tight plots or credible scenarios, but to avoid descending completely into farce they have always had at least a passing acquaintance with reality. The world of James Bond has always been known for its heavy reliance on technology and science, even if this has been in the form of outlandish plots to take over the world or improbable gadgets that allow our hero to defeat the baddies. However, at the heart of it all, there have always been some grains of scientific truth and references to the scientific preoccupations of the age. The fictional films have on occasion paralleled real-life stories of spies being poisoned and global threats from nuclear or biological weapons. And, although it may not be the first thing that springs to mind when someone mentions 007, chemistry has featured in a lot of the films, and not just in the cocktails that the British spy quaffs at an alarming rate. So just in time for the latest installment, this is a celebration of a few famous chemical moments from the 007 universe.
World domination through natural products
Bond villains are never lacking in ambition but Hugo Drax takes evil genius to a whole new level. He doesn’t want gold, or power, he wants everything. His diabolical plan is to wipe out the entire human race and start over again with his specially chosen group of beautiful people. To do this he has isolated a harmful chemical from an orchid and engineered it into a killer compound that he plans to disperse over the entire globe from the comfort of his secret space station.

The plot of Moonraker is perhaps the most outlandish of all of the films. But forgetting the more ridiculous aspects of the plot, like space lasers and metal teeth that can bite through steel cables, methods to kill huge numbers of people using chemicals extracted from plants is sadly a lot closer to the real world than we might like to admit.
Drax, or his scientists, have extracted a pharmacologically active component from an orchid and modified it to his purpose. This is no different to pharmaceutical companies taking plant extracts and chemically manipulating them into lifesaving drugs, such as 10-deactylbaccatin extracted from European yews and converted into anticancer drug Taxol. Drax is not interested in saving lives, however: he has modified the orchid extract to enhance its toxic properties. Drax is certainly more ambitious in scale but developing toxins for dispersal over large areas is sadly nothing new.
The large scale lethality of ricin, a protein extracted from castor oil plants (Ricinis comunis), was first investigated during the first world war by the US. Ricin is exceptionally toxic in tiny quantities if it is introduced into the bloodstream and worryingly dangerous if powdered ricin is inhaled, but it is much less dangerous if eaten. Being a protein it is easily denatured by heat or an individual’s digestive system. Conventional bombs can’t be used for ricin dispersal as they generate too much heat. Adding it to food or the water supply would require huge quantities. Eventually the US decided phosgene gas (COCl2) was more suited to their needs.
The film glosses over the details of how an enormous space station could be built without anyone noticing
Interest in ricin was revived during the second world war, but this time France and the UK also got involved. France decided ricin was too dangerous to work with until an antidote had been developed. The UK seemed less concerned with antidotes and got as far as developing bomblets that spread clouds of ricin dust that could be inhaled. The US developed ways of producing ricin on a huge scale and grinding it using specially chilled milling machines to prevent the heat of friction from denaturing it. These considerable technological advances meant ricin could be mass produced and dispersed effectively, but they still couldn’t overcome ricin’s considerable disadvantages.
Ricin will hang around in the environment for days until chemical bonds in its structure are broken by UV light. In the meantime, particles of ricin dust could stick to clothes and be inhaled by the wearer. Most importantly, ricin couldn’t distinguish between friend or foe. Without an antidote, the only practical way of using ricin would be disperse it remotely, and keep yourself at a safe distance for several days. A secret space station and bombs dropped from orbit would be the ideal scenario, but beyond second world war technology or budgets. Ricin plans were dropped.
In the fictional world of James Bond and Hugo Drax, advanced technology and sky-high costs are only minor hurdles to be overcome. The film glosses over the details of how an enormous space station could be built without anyone noticing, but there is quite a lot of information about the toxic compound Drax planned to release from it. There is even a chemical structure of the offending molecule shown in Q’s briefing to Bond. Don’t look too closely, there are a few errors in the structure, but the film makers deserve points for at least putting a structure on screen and trying to making it look credible. There are even fragments of the molecule that have a more than passing resemblance to lethal phosphine compounds (there is a diethyl phosphine oxide hanging off one end of the structure, an extra oxygen and it’s not too far off the nerve agent VX) and sections that, if you squint a bit, could be a steroid (three fused carbon rings in the middle of the molecule). It’s certainly a much better effort than a lot of other structures I’ve gasped at in horror in the darkness of a cinema. Many details in Moonraker stretch credibility to breaking point, but the general premise has at least some nuggets of truth at its heart.
A pointed argument
One of the most infamous weapons in the entire 007 series packs a powerful kick. The venom-tipped blade hidden in the Russian spy’s shoe may seem ridiculous but it is not as far-fetched as it sounds. For thousands of years poisons have been added to arrow heads and smeared on swords. Even in the twentieth century, with its considerable advances in technology, secret services around the world have tried to make their weaponry even more deadly by adding toxic chemicals to blades, bullets and umbrellas. Assassins have fired pellets filled with ricin into dissidents on the streets of Paris and London. The Nazis looked into adding cyanide to bullets to make them doubly lethal. But, as you might expect for the creator of James Bond, Ian Fleming made use of a more exotic poison.

The film version of From Russia With Love makes no mention of what compound has been smeared on the blades hidden in the Russians’ shoes, though it is described as a venom and Blofeld comments it takes a mere twelve seconds to kill. In the film Bond also manages to escape the Russian’s attacks on his shins. In the novel, Klebb makes contact with her lethal footwear. The book ends on a cliffhanger with the reader not knowing if 007 survives or not.
Now we can just pick up the next instalment in the saga but in 1957, when the book was published, fans had to wait another year to find out that Bond had been poisoned with tetrodotoxin. Luckily he survived, but it was a very narrow escape.
Tetrodotoxin is a powerful nerve toxin found in puffer fish, blue-ringed octopuses (later to encounter a henchman’s face in Octopussy) and a number of other animals. These animals do not produce the toxin themselves but accumulate it from a by-product produced by bacteria in their environment. Just a few milligrams can be fatal to humans because it disrupts the function of sodium channels in neurons. These channels allow sodium ions to flow into the cell to generate the electrical signal needed to pass on messages in the body. When not in use the nerve is primed ready for action by expelling the sodium ions and shutting the channel behind them. By blocking the sodium channel, tetrodotoxin stops the sodium ions from entering the cell when needed and the nerve cannot fire. The result is paralysis, and with a sufficient dose, death by asphyxiation as the muscles that control breathing cannot work. There is no antidote but artificial support for breathing can allow the patient to survive until the toxin can be cleared naturally from the body.
Bond survived Klebb’s assault because he was given mouth to mouth resuscitation to keep him breathing until a doctor came. Then another stroke of luck, the doctor treating him had spent time in South America and thought Bond’s paralysis had been caused by curare, another nerve poison that causes paralysis, and treated him accordingly. The henchman in Octopussy with the octopus stuck to his face was not so fortunate.
In the drink
Something slipped into a drink is perhaps the oldest trick in the book and it may be surprising that Bond falls for it. But considering the real life death of Alexander Litvinenko was brought about by something added in his tea, maybe it isn’t ridiculous at all. Casino Royale was released the same month Litvinenko was poisoned. The method may have been uncomfortably similar, but the poisons were very different.

Polonium-210 was a very new poison in 2006. Litvinenko’s case was the first known use of polonium in this way and was only identified after his death. Even if it had been suspected from the start, there was tragically little that could have been done to save his life. In the fictional world of James Bond, the writers went for a compound that had been known about for centuries and, crucially for the plot, one where an overdose could be treated rapidly and effectively.
The baddies in Bond don’t seem to have troubled themselves with the tricky separation of a single compound
Digitalis is a group of very similar compounds found in foxglove plants. Individual components of digitalis, such as digitoxin, are vital medicines in the treatment of a number of heart conditions. But the baddies in Bond don’t seem to have troubled themselves with the tricky separation of a single compound and have added plain old digitalis to 007’s cocktail. We see it produce the symptoms you would expect for an overdose: nausea, sweating and the disrupted heart rhythm that is what kills in a digitalis overdose.
Digitalis compounds, such as digoxin and digitoxin, interact with Na+/K+-ATPase, a molecule involved in regulating nerve signals, which also indirectly controls the amount of calcium ions in a cell. More calcium means the cells contract more strongly. These compounds, therefore, alter the electrical signals sent by the nerves that coordinate the contractions of the heart, and the strength of those contractions through the movement of calcium ions. In appropriate doses it corrects irregularities and allows efficient pumping of the blood around the body. Too much digitalis and nerve signals become chaotic and the heart cells can contract more and more until it becomes paralysed.
The novocaine Bond injects in the film would be the most effective immediate treatment for a digitalis overdose, but the shocks from a defibrillator are a bad idea. The jolts of electricity can further complicate the electrical signals in the heart, and even stop it altogether. However, it does make for a dramatic scene and serves the plot extremely well as it is Vesper, Bond’s love interest, who delivers the jolt.
The clichéd capsule
The poison most commonly associated with spies, the cyanide capsule, features in several Bond films. It is the quick way out for a spy or henchman who has been caught and doesn’t want to divulge important secrets. The now clichéd capsule made its first appearance in the first twenty minutes of the first Bond film, Dr. No. It was hidden in a cigarette used by an enemy agent to escape Bond’s clutches. Bond was able to identify the poison almost immediately, by the rapid death and characteristic smell of cyanide. Since then, cyanide has made repeat appearances in the franchise.
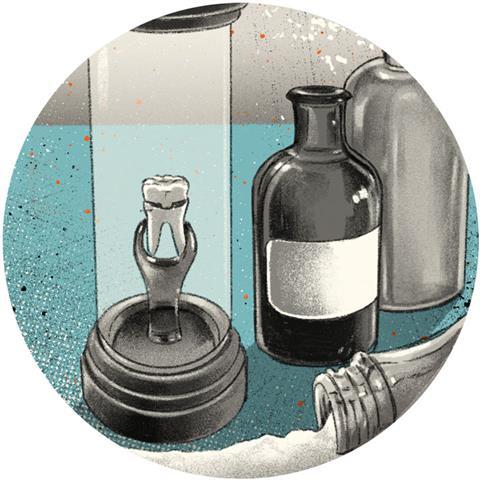
One of the most memorable cyanide scenes is from Skyfall during Raoul Silva’s confrontation with M. He explains how when he was captured, he had attempted to use the standard-issue cyanide capsule hidden in his tooth, but that the poison had failed to kill him. Instead, it had left him horribly disfigured. To prove his point he pulls a prosthetic out of his mouth revealing extensive damage to the bone. As usual for the 007 series, there is a kernel of truth that has been exploited and stretched to make a dramatic cinematic scene.
Extensive damage to skull bones from cyanide is highly unlikely but does make for a very good special effect
That cyanide pills existed and were given to agents is based on historic fact. During the second world war L-pills were given to agents on top secret missions. These were glass pills that contained a lethal amount of cyanide. Biting down on the pill would break the glass and release the poison. Cyanide is easily absorbed into the body and readily diffuses into cells where it blocks cytochrome oxidase enzymes. This prevents the respiration process and the cells die. It would be a very unpleasant way to go, with blinding headaches, vomiting, convulsions and coma, but it would be over in a few minutes. That Silva expected the cyanide to kill him is therefore credible but the damage to his face is where science starts to be stretched perhaps a little too far.
The cyanide in the pill would have been either the potassium or sodium salt. When it came into contact with moisture in the mouth it would hydrolyse to produce hydrocyanic acid. Acids certainly have the potential to react with calcium carbonate in bones but hydrocyanic acid is very weak, weaker even than acetic acid in vinegar. To be fair, the acetic acid in vinegar is very dilute and the cyanide in Silva’s capsule would have been very concentrated. Damage to the soft tissues inside the mouth would not be unexpected. Extensive damage to skull bones is highly unlikely but does make for a very good special effect. It also puts doubts in the viewers’ minds that maybe M got it wrong and Silva has been badly treated by the service. It set up a more complex relationship between the good guys and the bad guys than had previously been seen in the 007’s world.
There has been a lot of speculation about the plot and characters that will appear in the new James Bond film. But wherever the secret agent takes us, we can expect amazing action sequences, big explosions – and maybe even some interesting science.
Kathryn Harkup is a science writer based in Guildford, UK














2 readers' comments