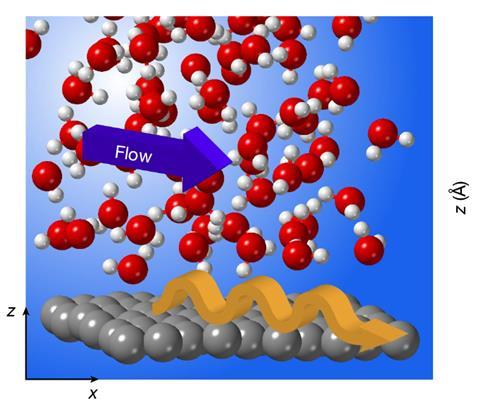
‘Quantum friction’ – a component of friction not present in classical physics, has been postulated by researchers in France to explain anomalies in the behaviour of water at carbon-based surfaces like graphite, graphene and carbon nanotubes. The phenomenon’s generality is not yet known, but it could potentially be useful in fields such as desalination that require selectively permeable membranes.
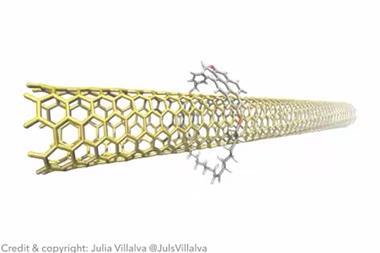
At macroscopic scales, the flow rate of a liquid past a solid is usually approximated to be zero at the interface, making the overall flow dependent only on the liquid’s viscosity. However, on the nanoscale, when only a very thin liquid layer flows, the liquid–solid friction becomes crucial. Traditionally, scientists think of friction as the result of the roughness of solid surfaces. However, this fails to explain some odd experimental observations: notably, water flows through microfluidic channels in graphene much faster than in bulk graphite. More bizarrely, in 2016 Lydéric Bocquet and colleagues at Ecole Normale Supérieure in Paris found that, when multi-walled carbon nanotubes become narrower, water actually flows through them faster.
Researchers have tried to explain these observations using more sophisticated models of friction. Electronic friction considers the way electronic excitations in a solid surface can resist motion. ‘Typically the idea is that you have a single charged particle moving next to an electron gas, and this charged particle’s Coulomb potential creates excitations that give rise to friction,’ explains Nikita Kavokine, who has just left Bocquet’s group to join the Flatiron Institute in the US. Researchers, such as condensed matter theorist Jeffrey Sokoloff of Northeastern University in Massachusetts, tried to apply this concept to flowing water. ‘The problem is that if you take liquid water on average, it is neutral and there is no charge … so you need to consider all the water molecules undergoing their random thermal motions,’ explains Kavokine.
In the new work from Bocquet’s group, Kavokine and colleagues describe these collective charge fluctuations in flowing water as quantised excitations called ‘hydrons’. These hydrons cannot dissipate significant amounts of energy into graphene, the researchers calculate, so only classical friction is important. When water flows past bulk graphite, however, hydrons resonantly excite quantised electron density fluctuations called plasmons, thereby slowing down the water. ‘Graphite has this very peculiar surface plasmon that has precisely the right energy and momentum to talk to the hydrons,’ explains Kavokine. ‘Water friction is not anomalously low on graphene, but instead it is anomalously high on graphite,’ the researchers write. In this case, the quantum friction can be about 10 times as large as the classical friction.
Mildred Dresselhaus and colleagues showed back in 1997 – before graphene was even isolated – that large multi-walled carbon nanotubes have a graphite-like structure, whereas smaller nanotubes are more like concentric sheets of graphene. This decoupling, Bocquet’s group shows, can naturally explain the counter-intuitive 2016 results.
The researchers are now working to understand the fundamental physics of quantum friction better and to see if it applies in other systems. ‘I think we’re going to be able to understand many more strange phenomena that happen at interfaces using these theoretical tools,’ says Kavokine. They are now looking to develop applications. ‘I cannot disclose completely what we have in mind but this can really be an asset in filtration and things like that,’ Bocquet says.
‘It’s a reasonable explanation for an important experimental result,’ says Sokoloff. ‘Flow of water in carbon nanotubes is very important because it has possible applications in things like water desalination… [The researchers] will only have truly solved the problem if their mechanism explains future experiments – which they are working on, and maybe other groups are too. But they seem to be the ones best set up to do these experiments, so they’re basically explaining their own experiment.’
References
N Kavokine, ML Bocquet and L Bocquet, Nature, 2022, 602, 84 (DOI: 10.1038/s41586-021-04284-7)












No comments yet