Lithium–air batteries hold the promise of great enough power density to fuel cars – but has their progress stalled in recent years? Philip Ball finds out
Lithium–air batteries hold the promise of great enough power density to fuel cars – but has their progress stalled in recent years? Philip Ball finds out

You know the feeling: just when you need to make that crucial phone call, your mobile phone’s battery is flat. But now imagine you are driving through the middle of nowhere on a wet night, you still have 300 miles to go, and the alarming ‘low battery’ sign is flashing not on your phone but on the dashboard of your electric car.
With fuel costs soaring and pollution and global warming worsening, many people like electric cars – in principle. But in practice they are deterred by ‘range anxiety’: the fear of being stranded without power. A full tank of petrol in a family car might get you 400 miles, but today’s electric vehicles rarely manage a quarter of that, and charging takes hours. So they are fairly hopeless for long journeys.
IBM, now diversifying from information to energy technologies, hopes to crack the electric-vehicle range problem with its Battery 500 project. Launched in 2009, it aims to develop a battery capable of powering a car for 500 miles with a single charge. And like many other battery researchers, it is putting its faith in a battery that could boost the energy capacity to more than 10 times what existing batteries can achieve. This battery runs on air – more specifically, on the energetic reaction of oxygen with lithium. With lithium’s low density, its oxidation can in theory provide an energy density (energy release per kilogram) much higher than the batteries currently in common use for electric vehicles (primarily nickel–metal hydride and lithium-ion), and comparable to that of petrol.1
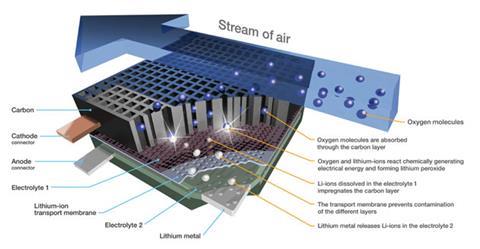
But the obstacles to making lithium–air batteries a practical technology for vehicles are daunting.2 They have to be cheap, safe, rechargeable and able to withstand many charge–discharge cycles. ‘Safety, range and cost are the key items limiting the development of electric cars,’ says the Battery 500 project’s manager, Winfried Wilcke of IBM’s Almaden research laboratory in San Jose, California, where the initiative is based.
The challenges are complicated by the fact that, although prototype lithium–air batteries have been developed, no one is yet entirely sure what the electrochemical processes at the electrodes are, and this interface between electrolyte and electrode is evidently complicated and hard to control. ‘We need to precisely understand what chemistry is happening inside the cells so that they can be designed with the appropriate materials,’ says Wilcke. And that is proving tricky.
Air power
The lithium–air battery was first proposed in the 1970s for use in electric vehicles, but there was little serious work to develop it until 1996. That is when Kuzhikalail Abraham of the research and development company EIC Laboratories in Norwood, Massachusetts, reported a non-aqueous rechargeable device with a lithium metal anode, a porous carbon cathode and a polymer gel electrolyte.3
Making thin and robust membranes is challenging
At the anode, lithium atoms lose an electron to form ions that pass into the electrolyte containing a dissolved lithium salt. At the cathode, air – or in this case, and most other devices made so far, pure oxygen – diffuses into an electrode made from electrically conducting porous carbon, and the oxygen is reduced, reacting with lithium ions to form insoluble lithium peroxide, Li2O2 (see box below). When the battery is recharged, this compound, deposited on the carbon surface, dissolves with the release of lithium ions and oxygen gas. For the next 15 years, these were assumed to be the key electrode reactions. Recently, it has become clear that the story is not so simple.
Abrahams’ ‘aprotic’ cell – so-called because it uses a solvent with no easily ionisable hydrogen ions – remains the most promising design, but it is not unique. Lithium–air batteries with an aqueous electrolyte have also been made, and have the advantage that the product formed at the cathode is a soluble lithium compound (usually lithium hydroxide), which avoids clogging the interface (see box below). But in that case, the lithium anode must be coated with a barrier layer that allows lithium ions to pass through, while protecting the metal from reacting with water. Besides, it has not been possible so far to make the aqueous reactions reversible: these would be one-shot batteries, useless for vehicles. That is why most of the focus is currently on aprotic cells.
Material differences
In some ways, the aprotic lithium–air battery is very similar to the first lithium-ion batteries, in which a lithium anode was also converted to lithium ions during discharge. These were then ferried by a non-aqueous electrolyte, such as propylene carbonate, to a cathode made from a lithium-intercalating material, such as lithium manganese oxide. The difference is that the cathode reaction in the air battery is much more energetic, producing a much higher energy density with rather similar materials. But those early lithium batteries became notorious because of their tendency to burst into flames; the anode was prone to corrode and to react with the electrolyte. And during the recharging cycle, the lithium metal was not necessarily redeposited in a smooth coating, but could grow into branching fingers called dendrites, which eventually caused the device to short-circuit.
Lithium–air batteries face the same problems. Lithium anodes react with the solvent, and perhaps with the complex organic anions of the lithium salt, to form a range of compounds that coat and ‘passivate’ the surface, preventing further reaction and creating a stable interface. They work, but it is not very clear why – the resulting surface is non-uniform, brittle and chemically diverse.
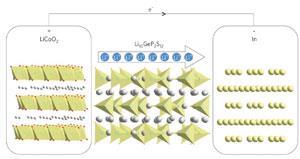
One approach to achieving greater control of the anode surface has been to coat the lithium with a protective layer that can transmit lithium ions, such as so-called lithium superionic ceramics. Last year, researchers at Toyota teamed up with scientists at the Toyko Institute of Technology to develop a material, Li10GeP2S12, with a very high lithium-ion conductivity, and demonstrated its use in a lithium-ion battery.4 Unfortunately, these ceramics might themselves be liable to react with lithium. So a further protective thin film of a more stable lithium compound, like lithium nitride or phosphide, must be inserted between the electrode and the thicker superionic conductor. That makes for a rather complex interface and manufacturing process. ‘The conductivities of these protective layers are generally low, and making thin, mechanically robust membranes is challenging,’ says Jake Christensen, an engineer at the Bosch Research and Technology Center in Palo Alto, California.
There is plenty to worry about at the cathode too. Here, lithium ions can react reductively with oxygen to form a variety of products, depending on the conditions – it is likely, for example, that lithium superoxide, LiO2, might be formed as well as the peroxide. The buildup of these insoluble compounds can inhibit the flow of reactants to the electrode surface, where they pick up electrons – not just by creating an insulating coating on the porous carbon surface, but by clogging the pores themselves and reducing the accessible surface area. These problems are thought to be one reason why a higher voltage is needed to charge the battery than it produces during discharge.
Potential problems
Around five years ago, it was found that this so-called overpotential can be reduced by placing catalysts on the cathode surface, such as metal nanoparticles or oxides such as manganese oxide. It was thought that one of the roles of these catalysts is to speed up the rate with which the molecular oxygen is reduced at the electrode surface. But it seems that this alleged catalysis may have been a red herring.
‘Papers that showed a decrease in overpotential in cells with catalysts such as gold and platinum erroneously concluded that catalysis of the desired discharge–charge reactions was happening,’ says Christensen. ‘However, it turned out that these catalysts were simply decomposing the electrolyte more effectively. The IBM group has shown pretty definitively that electrocatalysis plays absolutely no role in aprotic lithium–air cells.’
Although Peter Bruce at the University of St Andrews in Scotland agrees that catalysts were mainly just agents of solvent decomposition in the earlier work, he feels it is too early to exclude a potentially more useful role for them. ‘We are only now accessing electrolytes and electrodes with sufficient stability to explore this issue,’ he says.
With catalysts or without, degradation of the electrolyte is a serious problem. In late 2009, a team at Toyota’s Battery Research Division in Susono, Japan, found that their lithium–air battery with a propylene carbonate electrolyte was not producing lithium oxides at the cathode at all.5 The researchers figured that the lithium ions were instead reacting with the electrolyte.
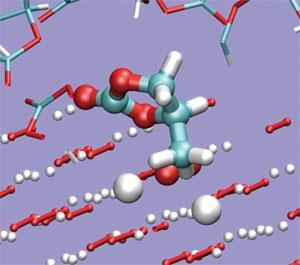
Last year, Bruce and colleagues showed that these reactions produce a range of different lithium carbonate compounds, as well as carbon dioxide.6 Earlier this year, Alessandro Curioni and Teodoro Laino of IBM’s Zurich Research Laboratory clarified how this happens. Their ab initio molecular dynamics simulations of the reactivity of Li2O2 and propylene carbonate show that the peroxide is considerably more reactive in solid form than as a solution-phase single unit, decomposing the carbonate solvent to an alkyl carbonate.7
A battery that eats itself is clearly not going to go far – these devices will need more oxidation-resistant electrolytes. ‘Once you know the type of chemistry behind this instability, it’s relatively easy to screen for new solvents,’ says Curioni. ‘That’s what we’re now doing.’ However, although other solvents, such as dimethoxyethane and ethers, have been tried, none has shown full reversibility – that is, no side-reactions, so that the cell can be returned during charging to the same state in which it started. ‘We still have unwanted side reactions going on, and our main goal is to eliminate these by improving the electrolytes further,’ says Wilcke.
Some researchers believe that ionic liquids – salts that are liquid at room temperature, which usually contain large organic ions – might provide the answer. Although those currently known also tend to be degraded at large electrochemical potentials, several groups are working towards making them more robust. The problem of solvent degradation might also be mitigated by careful choice of the cathode material.
While most cells explored so far use porous carbon, Bruce and colleagues recently found that the decomposition of a dimethyl sulfoxide solvent can be avoided by using a nanoporous gold cathode.8 They show that the gold is much more effective than carbon at promoting the oxidation of Li2O2 during charging, although quite why that is – whether it is a form of catalysis, say – is not yet clear. Gold electrodes would be both heavy and expensive, but gold-coated porous carbon also works quite well. When it comes to cost, Bruce points out that ‘there is a significant amount of gold in every mobile phone. However, the first choice would be to find a low-cost alternative.’
Our main goal is to eliminate unwanted side reactions by improving the electrolytes
Nearly all of the prototype devices made so far have used pure oxygen rather than air, to avoid unwanted side reactions with water vapour, carbon dioxide and nitrogen. For any practical devices, the oxygen will first need to be extracted, or at least enriched. The battery could in fact be run with pure oxygen carried in an onboard tank – but that introduces a whole new set of constraints for the economics, infrastructure, safety, and weight of the vehicle. Wilcke believes that any practical device on a vehicle will have to use air rather than pure oxygen, although Christensen does not rule out the latter.
Water vapour is a particular problem: it tends always to pass through membranes more readily than oxygen, partly because the molecules are smaller, and it is of course highly corrosive towards lithium. Even hydrophobic ionic-liquid solvents9 don’t fully exclude water. Aishui Yu of Fudan University in Shanghai and colleagues have developed a firmer seal by mixing a hydrophobic ionic liquid with a hydrophobic polymer (a copolymer of polyvinylidene fluoride and polyhexafluoropropylene) and fine particles of hydrophobic (alkyl-coated) silica. This composite material allows oxygen to permeate while excluding water vapour, so that the device can work with ambient air without the metal anode being attacked by water.10
Test of time
Even if all these challenges can be met, lithium–air batteries have to be reliably rechargeable many times. Current prototypes typically show significantly poorer performance after just a few tens of charge–discharge cycles. Cyclability does not impose as big a demand as it might at first seem, since a battery with a 500-mile range would need recharging only around 300 times to attain a respectable lifetime mileage of 150,000 miles. All the same, if it is highly sensitive to moisture, a battery will not last long in a real vehicle.
Some new electric cars now use lithium-ion batteries: bigger versions of those in your laptop or mobile. However, it took about three decades for lithium-ion batteries to reach this stage, and lithium–air batteries are likely to require a similar period of intense research before they are ready to go under the bonnet. ‘If everything works well, we might have batteries ready for use in vehicles around 2025,’ says Wilcke.
That will need plenty of investment. ‘The level of funding for energy storage isn’t sufficient to meet the challenges that must be overcome,’ says Christensen. ‘We need significantly cheaper batteries to enable widespread adoption of electric vehicles, and it will be hard to get there with conventional lithium-ion cells.’ Lithium–air batteries do look like the best alternative – but it’s going to be a long journey.
Philip Ball is a science writer based in London, UK
Electrochemistry of the lithium–air battery
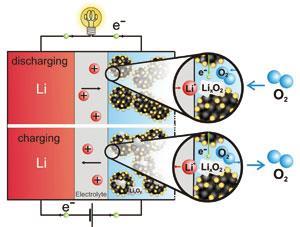
For an aprotic battery (using an organic solvent), lithium metal is oxidised at the anode:
Li ? Li+ + e–
At the cathode, the ions combine with molecular oxygen in a reduction process:
2Li+ + O2 + 2e– ? Li2O2 (s)
That, at least, is the theory. But in practice there are several possible products of the reaction of lithium ions and oxygen, especially if there are trace amounts of water present or if the solvent is insufficiently stable. And the peroxide can go on to react with the solvent.
Lithium–air batteries may also use aqueous solvents, in which case the cathode discharge reaction is:
2Li+ + ½O2 + H2O + 2e– ? 2LiOH
This reaction, in contrast to the aprotic case, demands cleavage of the O–O bond, so it seems that catalysts, such as platinum, at the cathode can be genuinely effective.
References
1 G Girishkumar et al, J. Phys. Chem. Lett., 2010, 1, 2193 (DOI: 10.1021/jz1005384)
2 J Christensen et al, J. Electrochem. Soc., 2012, 159, R1 (DOI: 10.1149/2.086202jes)
3 K M Abraham and Z Jiang, J. Electrochem. Soc., 1996, 143, 1 (DOI: 10.1149/1.1836378)
4 N Kamaya et al, Nat. Mater., 2011, 10, 682 (DOI: 10.1038/nmat3066)
5 F Mizuno et al, Electrochemistry, 2010, 78, 403
6 S Freunberger et al, J. Am. Chem. Soc., 2011, 133, 8040 (DOI: 10.1021/ja2021747)
7 T Laino and A Curioni, Chemistry, 2012, 18, 3510 (DOI: 10.1002/chem.201103057)
8 Z Peng et al, Science, 2012, 337, 563 (DOI: 10.1126/science.1223985)
9 T Kuboki et al, J. Power Sources, 2005, 146, 766 (DOI: 10.1016/j.jpowsour.2005.03.082)
10 D Zhang et al, J. Power Sources, 2010, 195, 1202 (DOI: 10.1016/j.jpowsour.2009.08.063)












No comments yet