Siloxane molecular wires could be a handy component for nanoscale electronics
A new type of molecular wire – formed from repeat units of silicon and oxygen – has been found to demonstrate the greatest resistance ever recorded, making them ideal insulators for molecular circuits.
In order for nanoscale electronics to progress, it requires both conductors and insulators. Traditionally, most research has focused on the development of increasingly efficient conductors. However, researchers from Denmark and the US have managed to develop the most insulating nanoscale material to date – siloxane wires.
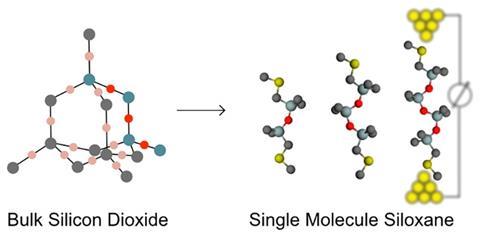
The conductance of the molecular wires, each a single molecule thick and up to 20Å long, was determined using the scanning tunnelling microscope-based break-junction method. When compared to alkanes, the prototypical molecular insulator, and silanes of the same length, siloxane wires were found to have a lower conductance than both.
Organic molecular materials containing silicon–oxygen bonds have already been found to exhibit promising dielectric properties, and it is thought that the gap between the highest occupied molecular orbital (HOMO) and the lowest occupied molecular orbital (LUMO) is at least partially responsible for siloxane’s lack of conductance. The calculated HOMO–LUMO gaps have been found to be essentially length independent, indicating weak electronic coupling across the backbone of the molecule. This promotes charge localisation, leading to their superior insulating properties.
References
L Haixing, J. Am. Chem. Soc., 2017, DOI: 10.1021/jacs.7b05599










No comments yet