This year’s chemistry Nobel prize has been awarded jointly to nanotechnology pioneers, Moungi Bawendi, Louis Brus, and Alexei Ekimov for the discovery and synthesis of quantum dots. However, news of their win was leaked around two hours before the official announcement was made, in a press release sent out in error to a Swedish newspaper by the Royal Swedish Academy of Sciences.
When asked about the mistake, Hans Ellegren, secretary general of the Royal Swedish Academy of Sciences, said it was ‘very unfortunate’ that the news was leaked. ‘We deeply regret what happened for sure, there was a press release sent out for still unknown reasons, we have been very active this morning to try to find out what happened but we don’t know … we deeply regret that it happened.’
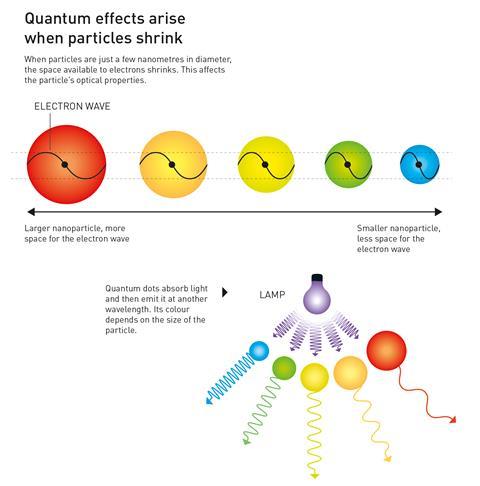
Quantum dots are semiconducting crystals just a few nanometres wide. They are typically made from combinations of transition metals and/or metalloids – most commonly cadmium selenide and cadmium telluride – just like regular semiconductors.
Changing the size of a quantum dot changes its properties, in particular its fluorescence, meaning they can be tuned to produce different colours. The smaller the nanoparticle, the narrower the wavelength it emits – smaller dots emit blue light while larger ones emit red light.
In 1981, Ekimov a solid-state physicist working at the time at the Vavilov State Optical Institute in Russia, succeeded in creating size-dependent quantum effects in coloured glass. The colour came from nanoparticles of copper chloride and Ekimov demonstrated that the particle size affected the colour of the glass via quantum effects. This was the first time someone had succeeded in deliberately producing quantum dots.
A few years later, unaware of Ekimov’s study, Brus, a US chemist studying colloidal semiconductor nanocrystals at AT&T Bell Laboratories, became the first scientist to prove size-dependent quantum effects in particles floating freely in a solution. He too understood that he had observed a size-dependent quantum effect.
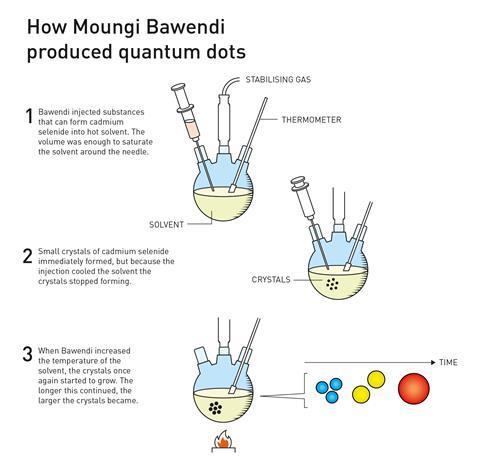
Continuing with efforts to produce higher quality nanoparticles, in 1993 Bawendi, a research leader at the Massachusetts Institute of Technology in the US, had a major breakthrough when his group succeeded in growing nanocrystals of a specific size. He was credited with revolutionising the chemical production of quantum dots, resulting in almost perfect particles – an essential step for them to then be mass produced.
During the press conference, a phone call was made to Bawendi in which he described the win as ‘an honour’. ‘I was very surprised, sleepy, shocked, unexpected, but very honoured,’ he said.
Responding to questions from journalists about the win and the implications of his work he said: ‘It’s a field with a lot of people that have contributed to it from the beginning… I didn’t think that it would be me that would get this prize because we’re all working together on this.
"This is a collaborative effort.”
— The Nobel Prize (@NobelPrize) October 4, 2023
For Louis Brus the award of his 2023 Nobel Prize in Chemistry has been a team effort. Listen to our interview with him where he pays tribute to his many collaborators. pic.twitter.com/huvWS10zTQ
‘This was a process, I think that the community, because it became a larger community, realised the implications in the mid-90s that there could potentially be some real-world applications.’

Now quantum dots are found in a wide range of commercial products, across scientific disciplines, from computer monitors and television screens based on QLED technology, to biomedical imaging.
‘Research is still very intense and there are applications expected or being heavily researched in producing photons for quantum communication, for flexible electronics for sensors for improving solar cells, making them better or cheaper … among many other things,’ said Heiner Linke, member of the Nobel committee for chemistry during the prize press conference.
‘So for these reasons, the Nobel prize in chemistry for 2023 will be awarded for to Alexei Ekimov and Louis Brus for the discovery that it is possible to make such quantum dots, and Moungi Bawendi for a synthesis method that made quantum dots widely useful.’
Gill Reid, president of the Royal Society of Chemistry, said the recognition of this work on quantum dots was ‘really exciting’ as it showed how chemistry could be used to solve a range of challenges.
‘These remarkable nanoparticles have huge potential to create smaller, faster, smarter devices, increasing the efficiency of solar panels and the brilliance of your TV screen,’ she said.
“I woke up!”
— The Nobel Prize (@NobelPrize) October 4, 2023
What was the first thing Alexei Ekimov did when after he had been awarded the 2023 #NobelPrize in Chemistry?
Listen to his first reaction in our interview with him: pic.twitter.com/bfkxgZmGJL
‘Great science benefits from diverse viewpoints as part of a collective endeavour, and this year’s prize is a great example of that – people working in different labs, in different countries, approaching a problem from different angles. We don’t work in isolation in chemistry – teamwork is both a fundamentally important aspect of how science is actually done, and one of the most fun!’
‘Such high recognition of the chemistry to realise the quantum size effect in soluble, depositable particles – and recognition of these three kind and brilliant people who accomplished it, along with their students and postdocs – is really a wonderful event,’ says Emily Weiss, a materials scientist at Northwestern University, US. ’Thanks to the clear illumination of the electronic structure of these particles by Brus, Bawendi and Ekimov, the basic physical chemistry of quantum dots is simple enough to engage general chemistry students, yet deep and nuanced enough to sustain an ever-growing field of chemists, physicists and materials scientists 40 years later, along the way triggering development of new spectroscopies, syntheses, and analytical techniques, and exciting applications in display, energy conversion, bioimaging, and quantum computing.’
‘Semiconductor quantum dots, which can most conveniently be synthesised as light emitting colloidal nanoparticles in solution, have for quite some time appeared in textbooks as a colourful illustration of the “quantum confinement effect”,’ said Andrey Rogach, a photonics researcher at the City University of Hong Kong. ’They are already used as light emitting materials for biolabelling applications and, very prominently, in LEDs and TV displays, as well as light absorbers in solar cells and photodetectors. It is great news for all the colleagues to receive recognition of quantum dots with the Nobel prize in chemistry 2023. Heartful congratulations to Moungi, Louis and Alexei, representing the pioneers of this field!’
David Pendlebury, head of research analysis at the Institute for Scientific Information at Clarivate, which identified both Brus and Bawendi as Nobel-worthy in 2012 and 2020, respectively, said the researchers’ ‘incredible contributions to chemistry’ were reflected in their citation records. ‘Bawendi shows an astonishing 100,000+ citations in the Web of Science, and Brus 60,000+,’ he added.
Update: A comment from Emily Weiss and Andrey Rogach was added on 4 October 2023.
















No comments yet