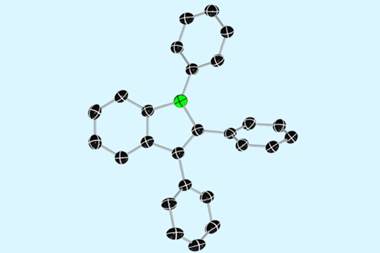
A rare example of an antiaromatic hydrocarbon that’s stable at room temperature has been made using a straightforward and completely reversible oxidation reaction. While annelation with other π systems is the most common way to stabilise an antiaromatic structure, 1,3,4,6-tetraphenylpentalene’s (Ph4Pn) stability appears to come from the steric bulk of its phenyl substituents.
The researchers synthesised Ph4Pn by oxidising Mg[Ph4Pn] with one equivalent of iodine, which created a MgI2 precipitate within minutes. NMR analysis of the filtrate confirmed it was a C2h symmetric pentalene with a localised olefinic π system. The pentalene exhibits remarkable stability. Variable temperature NMR studies conducted in THF showed no significant decomposition, however, the compound did decompose when exposed to air.
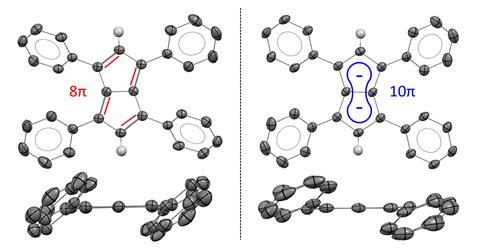
XRD analysis of Ph4Pn single crystals confirmed that its structure retains a bicyclic, co-planar pentalene core with no stacking observed in neither solid nor solution state. This means that the pentalene remains in a less stable, antiaromatic state without adopting a stacked 3D structure.
Ph4Pn can be fully reduced back into Ph4Pn2+ by treating it with a freshly prepared potassium mirror. This characteristic allows the hydrocarbon to switch between an aromatic and antiaromatic state without undergoing conformational or skeletal rearrangements.
The easy and reversible synthesis combined with the unique antiaromatic features of Ph4Pn could see the molecule and its derivatives have applications in optoelectronics.
References
This article is open access
H J Sanderson et al, Chem. Sci., 2025, DOI: 10.1039/d4sc06439a










1 Reader's comment