Researchers in the UK and US have obtained crystallographic evidence for global aromaticity and anti-aromaticity in reduced cyclophane hydrocarbons.
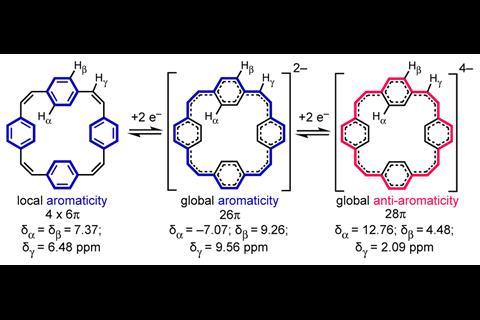
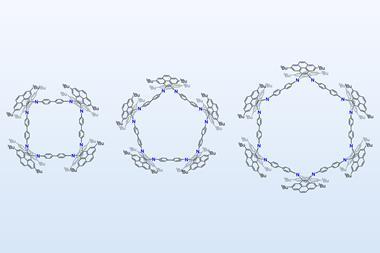
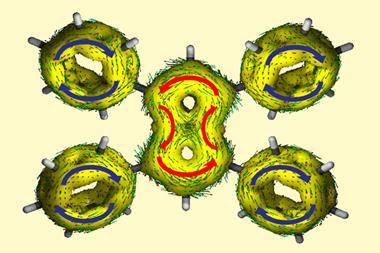
When [24]paracyclophanetetraene, a hoop of four benzene rings bridged by carbon–carbon double bonds, is in the neutral state, each benzene ring has its own distinct localised π-system. However, when reduced to the di-anion form, electron flow is no longer localised and instead circles the entire molecule. Further reducing it to the tetra-anion makes it anti-aromatic.
Chemists have recognised global aromaticity in the [24]paracyclophanetetraene di-anion for 40 years. [24]paracyclophanetetraene was one of the first compounds known to be able to switch from having local to global aromaticity and is an often-used example of a structure that can change from being aromatic to anti-aromatic. However, thus far the evidence of this was based on NMR experiments alone. Due to difficulties in crystallising the di-anion and tetra-anions, no one had previously investigated their solid-state structures. ‘[You] start to realise there are some basic things that have never been done,’ says Harry Anderson at the University of Oxford.
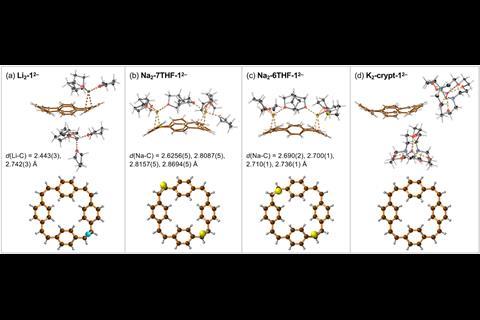
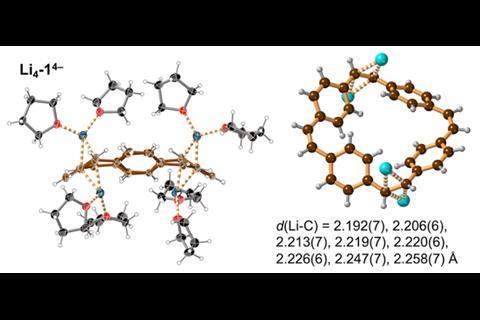
Now, by characterising the di- and tetra-anions of [24]paracyclophanetetraene in the solid state Anderson and colleagues have bond length, planarity and charge localisation data that confirms the structures are globally aromatic and anti-aromatic, respectively.
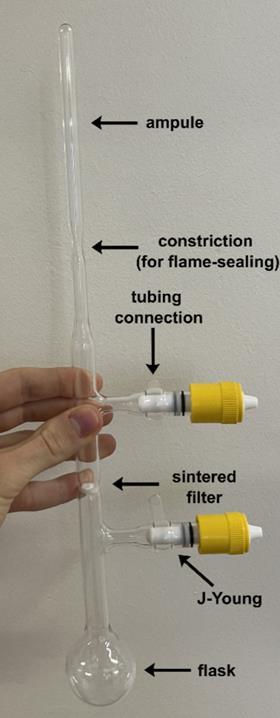
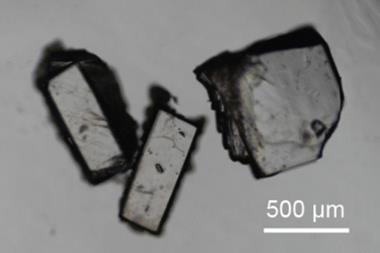
Anderson’s group collaborated with Marina Petrukhina and her team at the University of Albany in the US, who are experts in crystallisation. Anderson group member Wojciech Stawski travelled to Albany to work on the challenging crystallisation and says during their initial attempts ‘you could literally see with your bare eyes when there was some problem with oxygen or with moisture.’ They therefore had to use complex techniques, such as flame sealed glass ampules, to successfully crystallise the reduced cyclophane hydrocarbons.
Some of the team’s findings were not as expected. ‘Slightly paradoxical is that the evidence for the anti-aromaticity is partly that [24]paracyclophanetetraene distorts to prevent itself from being anti-aromatic,’ says Anderson. However, the distorted structure did not stop t he anti-aromatic behaviour and was in itself further evidence of anti-aromaticity.
Miquel Solà, an aromaticity expert at the University of Girona in Spain, says the work ‘represents a great contribution to the understanding of how aromaticity of macrocycles changes upon reduction.’ Solà however also notes that there are alternative interpretations of the work and that migrating Clar’s sextets may be responsible for some of the team’s observations .








No comments yet