Reaction that splits biphenols in two could convert lignin into useful chemicals
A reaction that cuts the hardy carbon–carbon bond between aromatic rings could be used to make valuable chemicals out of lignin, the major polymer in wood.
Lignin gives wood its rigid structure. Although chemists would like to be able to transform wood into fuels or other useful chemicals, lignin proved very resilient to being chopped up into smaller molecules. A team around Guangbin Dong from the University of Chicago, US, has now designed a bond-breaking reaction that might be able to do just that.
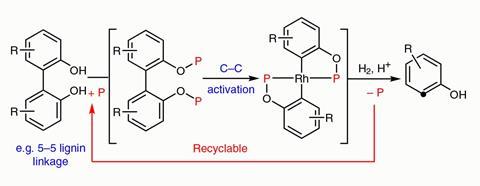
Their reaction breaks the aryl–aryl bond in 2,2’-biphenols. While there are reactions that cut strained or polarised carbon–carbon bonds, this is the first one to cleave unstrained biaryls. The connection between aromatic rings is stronger than many other C–C bonds and the twist angle between the rings makes it difficult for catalysts to access it.
Dong’s team first converts the biphenols into phosphinites. This allows a rhodium catalyst, introduced at the next stage, to grab hold of the compound and cut the carbon–carbon bond connecting the aromatic rings. The reaction runs under hydrogen, which the catalyst then adds to the aromatic rings’ loose ends.
The reaction sequence is valuable for making 2,3,4-trisubstituted phenols. These are usually difficult to synthesise due to the way the phenol influences where other substituents end up during electrophilic substitution.
The chemists also showed that they could convert wet spruce sawdust into dihydroeugenol, a lignin monomer, in a two-step procedure. First, hydrogenation converts lignin’s dibenzodioxocin units into biphenols, which are then converted into phosphinites and finally chopped up into the monomers by rhodium catalysis.
However, the researchers stress that this process is only a proof of concept. Since the reactions need to be carried out in an oxygen-free environment – Dong’s team uses a glove box – they might be difficult to scale up. Moreover, making the phosphinite requires a stoichiometric amount of phosphorus reagent – although the reagent can be recovered and recycled.
References
J Zhu, J Wang and G Dong, Nat. Chem., 2018, DOI: 10.1038/s41557-018-0157-x

















No comments yet