Carbon rings weaved into solvent-cleaning mesh
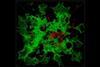
Tiny pores in conjugated carbon network cleanse organic solvents of impurities
A membrane constructed from π-conjugated carbon rings sieves impurities out of methanol, hexane and other organic solvents.