Researchers in China have devised electrocatalytic nanomaterials consisting of vanadium oxide cores within a vanadium nitride shell for reducing nitrogen to ammonia. The team found they could tune the nanomaterials’ core-shell structure to optimise the process’ efficiency and yield.
As one of the most produced inorganic chemicals, ammonia plays an important role in agriculture (for fertilisers), chemical synthesis and, more recently, as a hydrogen energy storage medium. Unlike the Haber–Bosch process, electrochemical nitrogen reduction can synthesise ammonia under ambient conditions and is therefore being investigated as a sustainable alternative.
To reduce nitrogen to ammonia electrochemically, an electrocatalyst is required. The electrocatalyst facilitates nitrogen adsorption and activation to overcome the strong N≡N triple bond. It also improves the reaction kinetics of the six proton-coupled electron transfer steps to transform dinitrogen into ammonia, but a current bottleneck is making an electrocatalyst that has both high activity and selectivity for the nitrogen reduction reaction.
Attempting to balance activity and selectivity, Yu Wang at Chongqing University and his team have made a core–shell nanomaterial electrocatalyst with a vanadium oxide core and a vanadium nitride shell. The researchers sought to harness the properties of both materials; metal nitrides can easily initiate the nitrogen reduction reaction at the nitride sites, which can then be activated by adjacent electronegative oxygen atoms to release ammonia for catalysis. ‘The oxide core design ensures that the material is not deactivated for a long time,’ says Wang, since surface oxygen atoms are unstable in the electrochemical environment.
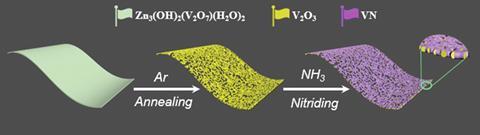
Wang’s team’s nanomaterial excelled at electrocatalytically reducing nitrogen to form ammonia in an acid electrolyte. The researchers obtained ammonia yields of up to 59.7mg/h (per milligram of catalyst) and faradaic efficiencies of up to 34.9%. Additionally, the electrocatalysts proved highly selective for ammonia production over hydrazine formation, and remained active after 50 hours of electrocatalysis with no structural changes.
Optimising the thickness of the nitride shell was an important aspect of the electrocatalyst design, especially relating to the d-band centre of the metal (which describes the interaction between the catalyst and adsorbate). Although the thinnest nitride shell system had the highest d-band and therefore the strongest interaction with dinitrogen as the adsorbate, the significant gap between the valence and conduction bands limits charge transfer and impedes its catalytic performance. The optimal, medium thickness nitride shell electrocatalyst strikes a balance with keeping the d-band centre high, whilst minimising the energy level difference between the valence and conduction bands for effective electron transfer and therefore electrocatalytic performance.
‘This work is close to the industrial ammonia faradaic efficiency limit,’ comments Ramendra Sundra Dey, from the Institute of Nano Science and Technology, Mohali in India. Dey says that the design principles for these core-shell nanomaterials could indeed be used to aim for higher faradaic efficiencies.
But Ib Chorkendorff, from the Villum Center for the Science of Sustainable Fuels and Chemicals at the Technical University of Denmark, is sceptical about the work. ‘This would be truly outstanding if true. However, I find the evidence for dinitrogen activation insufficient as the isotope experiments are not performed in a quantitative manner over several points.’
Wang agrees with that viewpoint and says the team is using IR and Raman techniques, as well as further isotope experiments, to study the N2 activation mechanism. ‘We hope that in our future work, we can have a profound interpretation of the N2 activation mechanism.’
References
This article is open access
X Yang et al, Chem. Sci., 2022, 13, 11030 (DOI: 10.1039/d2sc03975c)









1 Reader's comment