A newly developed battery based on aluminium, nitrogen and a specialised ionic liquid electrolyte can be used for energy storage via a net reaction that produces aluminium nitride by breaking the challenging nitrogen–nitrogen triple bond. Aluminium nitride can be converted to ammonia, opening a new avenue for coupling energy generation and the production of nitrogen-containing compounds, without relying on industrial routes that contribute to global warming.
Batteries based on gases can simultaneously store and release energy while also converting gaseous components to new compounds. These compounds may act as precursors to industrially relevant chemicals. For example, Li-CO2 batteries work by exploiting the electrochemical reduction of carbon dioxide to form a lithium carbonate. This carbonate can react further to create a range of different chemicals.
However, lithium is a somewhat costly metal that is also to difficult to handle. To overcome this limitation, a battery developed by the team of Chunyi Zhi at the City University of Hong Kong instead targets aluminium and nitrogen. This avoids the need for an expensive alkali metal anode and by incorporating nitrogen as the gaseous component, it provides a new route to ammonia that avoids the Haber–Bosch process.
Focusing on low-cost and naturally abundant aluminium as the metal anode allowed Zhi and co-workers to better engineer the underlying electrochemical reaction. This was achieved using an aluminium-based ionic liquid electrolyte instead of the ether-based electrolytes found in competing Li-N2 batteries. ‘The crucial point of the AlCl/1-butyl-3-methylimidazolium chloride electrolyte is its Lewis acidity, which enables the electroplating or stripping of aluminium on the anode because this process can only take place in acidic conditions,’ explains Zhi.
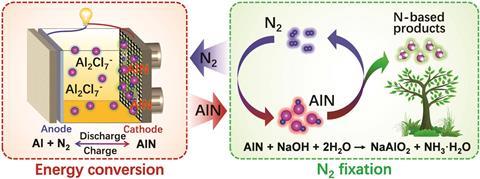
Using aluminium metal alongside this specific ionic liquid electrolyte allowed for a net reaction during battery discharge that involves reacting aluminium with nitrogen to afford aluminium nitride, a process that has a greater thermodynamic driving force than the production of Li3N in Li–N2 batteries. The resulting aluminium nitride, which forms at the cathode during battery operation, may then be reacted with sodium hydroxide, readily forming ammonia.
‘This is an interesting and more environmentally friendly alternative to the Haber–Bosch process to produce ammonia. Moreover, replacing lithium with aluminium in metal–nitrogen batteries would potentially reduce costs and increase the energy density of the battery,’ says Adriana Navarro-Suárez who researches electrochemical energy storage at Imperial College London, UK.
However, Navarro-Suárez notes an issue with the cathode and associated electrocatalyst within the battery. Currently, the cathode of the aluminium–nitrogen battery is based on graphene and palladium, the latter of which is a precious and costly metal. Yi-Chun Li, a battery specialist at the Chinese University of Hong Kong, also highlights this issue and while she notes that a battery which successfully harnesses nitrogen to produce aluminium nitride is impressive, ‘future work on replacing precious metal catalysts with other low-cost catalysts is important for large scale practical applications.’
Zhi and his team are actively considering this imposition to scale-up and widespread commercialisation. They intend to design special electrocatalysts based on other metals or metal oxides. They are also considering carbon-free catalysts and more stable electrolytes that would boost the energy conversion efficiency of the aluminium–nitrogen battery by avoiding parasitic reactions.
References
This article is free to acces until 14 October 2020
Y Guo et al, Energy Environ. Sci., 2020, DOI: 10.1039/d0ee01241f








1 Reader's comment