By monitoring an oscillating chemical system, researchers have observed clear early warning signals given off when the system nears the tipping point before it collapses into steady state behaviour.
A phenomenon called critical slowing down indicates a system is nearing a boundary between relative stability and potentially less familiar behaviour. Characterised by an increase in the time taken for the system to recover after being perturbed, this type of signal occurs in numerous systems, including ecosystems, global climate, infectious outbreaks, financial markets and the onset of depression. Now researchers have observed these same signals in a chemical network too.
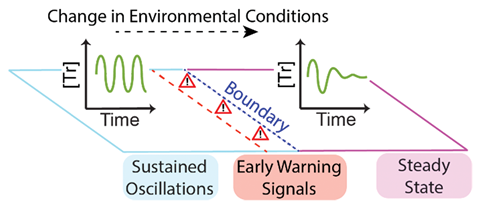
‘Our oscillating system has the smallest number of components necessary to produce oscillations in concentration over time,’ says Oliver Maguire, of Radboud University in the Netherlands who co-led the study. Their system, which was developed in a previous study, uses the enzyme trypsin and produces two possible behaviours depending on the temperature and flow rate: sustained oscillations in the trypsin concentration, or dampened oscillations that tend to a steady level of trypsin. Maguire and his colleagues temporarily raised the temperature of the system and measured how long it took to return to steady oscillations, in addition to monitoring the shape of the oscillation waves.
Close to the boundary between the two behaviours, the system displayed both critical slowing down behaviour (an increased recovery time following a perturbation) and a broadening of the oscillation waves. By monitoring for these signals, the group was able to show that their trypsin oscillator network displayed behaviour consistent with that predicted by models at different temperatures and flow-rates. ‘The importance of early warning signals was in part recognised because it allowed ecologists, climatologist and economists to identify when a system is close to collapse where it is not possible to fully model the system,’ explains Maguire.
In systems chemistry, for example, early warning signals have the potential to identify and correct parameters on the fly, thus increasing reaction stability. However, Maguire is careful to note that an application is still some way off. ‘In particular, we need to learn how to build chemical systems that can process information and signals, and then generate a response to this. Once we have this then we can look to integrate it into a feedback mechanism,’ he says.
Marten Scheffer of Wageningen University and Research in the Netherlands, who is interested in the stability and change of complex systems, is also positive about the potential of early warning signals in chemical systems. ‘In a sense it is not surprising that such generic early warning signals for critical transitions are now found for chemical reaction networks. We know by now that those dynamic signatures of resilience are generic enough to be discernible in a wide range of systems. However, it is exciting to see this being picked up in the field of chemistry where an entirely new range of applications may emerge.’
References
This article is open access
O R Maguire et al, Chem. Commun., 2020, DOI: 10.1039/d0cc01010c











No comments yet