Isolated germabenzenyl anion has aromatic character
A team of Japanese researchers has reduced germabenzene to produce the germanium analogue of a phenyl anion. The product was identified as a monomer both in solution and in solid state. It also appears to have aromatic character.
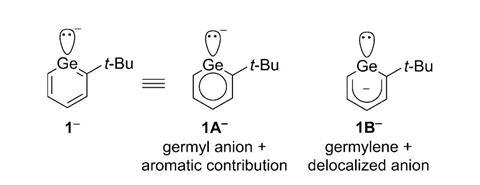
To prepare the elusive anion, chemists treated a germabenzene derivative with an excess of potassium graphite, one of the strongest reducing agents in existence. The reaction produced a pale yellow solid that turned out to be germabenzenyl potassium. The mechanism of the transformation is still unclear, however.
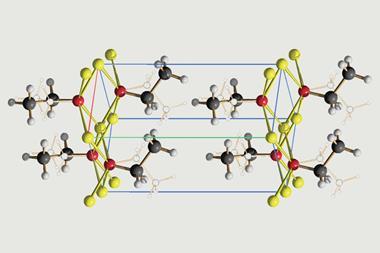
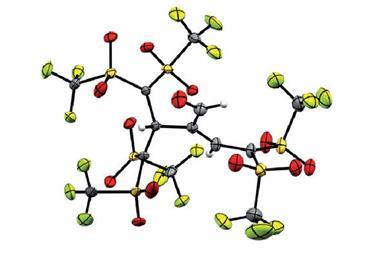
The team, led by Norihiro Tokitoh at Kyoto University, was able to fully characterise this new derivative. They obtained crystal structures that not only prove the existence of the germabenzenyl ion, but are also the first crystallographic analyses of arylpotassium compounds in the literature.
All the experiments, as well as detailed calculations, suggest that the germabenzenyl ion has a relatively aromatic character. However, it also has important contributions from ‘germylene’, a resonant structure bearing a delocalised negative charge in the ring. Although the presence of sterically bulky substituents may limit its synthetic applications, researchers believe they have found an interesting tool for unusual chemical reactions and new organogermanium compounds.
References
Y Mizuhata et al, Angew. Chem. Intl. Ed., 2017, DOI: 10.1002/anie.201700801













No comments yet