Tiny hydrocarbon with six double bonds has unrivalled ability to make complex ring systems though multiple Diels–Alder reactions
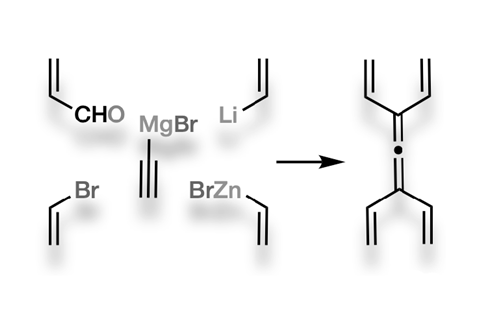
Chemists have made what might be the most double-bond-rich acyclic hydrocarbon ever synthesised: tetravinylallene. Made out of only 11 carbon atoms, the molecule packs a total of six double bonds. This gives it a surprising ability to create complex natural product structures in only a few steps.
Allenes are molecules with two adjacent double bonds sharing a common central carbon atom. They have proven useful for synthesising compounds with complex ring systems through multiple Diels–Alder reactions. In 2015, researchers used 1,1-divinylallene to cut the number of steps in the synthesis of the marine natural product pseudopterosin from 20 to 11.
A team of chemists from Australia has now for the first time made an allene that has over 50% more double bonds than divinylallene – although this wasn’t easy. It took ‘extensive experimentation’ to find conditions to make tetravinylallene in six steps.
Although tetravinylallene turned out to be unstable in air or when stored as a neat liquid, it could quickly make multi-ring structures better than its cousins divinylallene and dendralene. In only two synthetic steps comprising four pericyclic reactions, tetravinylallene produced four rings, seven carbon–carbon bonds and nine stereocentres.
Correction: This article was updated on 23 August to correct the number of carbon atoms in the molecule
References
C Elgindy, J S Ward and M S Sherburn, Angew. Chem. Int. Ed., 2019, DOI: 10.1002/anie.201908496
















No comments yet