The first magnesium-based electride has been synthesised by researchers in Norway, Poland and Spain. Electrides are of interest to materials scientists as they hold free-floating electrons in internal cavities, and therefore exhibit properties such as high conductivity. Among other things, this new species is stable at room temperature, offering new opportunities in materials science and catalysis.1
Thanks to their floating electrons, electrides have found applications in superconductors, optical materials, electronic devices and magnets.2 These free electrons also confer useful properties for catalysis, particularly in redox reactions such as the formation of ammonia from nitrogen and the valorisation of carbon dioxide. However, most of these species are complex inorganic mixtures. So far, eight organic electrides have been synthesised, and this magnesium-based example marks only the second species that’s stable at room temperature.
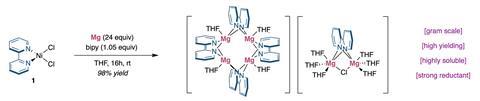
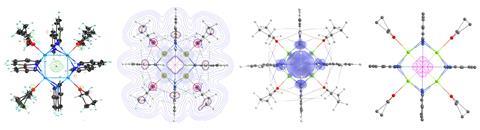
Moreover, the magnesium electride is easily prepared from an abundant nickel-dipyridine complex in just one step with excellent yields. The reaction leads to a tetra-nuclear magnesium compound, which holds a single electron in the cubic cavity formed by the metal atoms. Unlike other examples, in this case the electron isn’t delocalised over different cavities.
Researchers also tested the versatility of the new magnesium electride as a reducing agent. They attempted the synthesis of certain low-valent nickel complexes, which usually require the use of pure lithium and suffer undesired side-reactions. The reaction was quicker and cleaner, showcasing the potential of this magnesium electride in homogeneous catalysis. Moreover, the magnesium species is highly soluble in organic solvents and easily stored in a glovebox, which hints at promising possibilities in other reduction reactions.
References
1 CS Day et al, J. Am. Chem. Soc., 2022, 144, 13109 (DOI: 10.1021/jacs.2c01807)
2 X Zhang and G Yang, J. Phys. Chem. Lett., 2020, 11, 3841 (DOI: 10.1021/acs.jpclett.0c00671)














No comments yet