From nerve agent simulant, to pharma ingredient
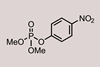
Portable microreactor offers a new way to dispose of paraoxon
Scientists in South Korea have shown how to transform a chemical warfare agent simulant into a common drug.

Portable microreactor offers a new way to dispose of paraoxon
Scientists in South Korea have shown how to transform a chemical warfare agent simulant into a common drug.