The first stable compound with a beryllium–beryllium bond, diberyllocene, has been isolated by researchers at the University of Oxford.
Due to the element’s toxicity, the chemistry of beryllium is one of the least understood of all the non-radioactive elements. The synthesis should help to answer some questions about the fundamental nature of beryllium–beryllium bonding – in particular its capacity for covalency – that were posed more than a century ago.
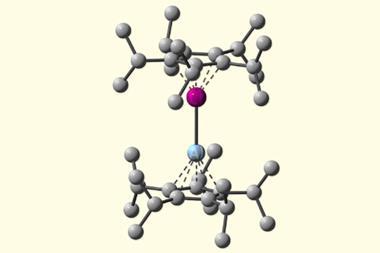
The researchers synthesised diberyllocene by reducing the organoberyllium sandwich compound beryllocene using a dimeric magnesium(i) complex. This complex has previously been used for the controlled reduction of a variety of organometallic compounds. The reaction was carried out at room temperature, under an inert atmosphere in toluene and the researchers showed that the synthesised diberyllocene was stable as both a solid and a gas.
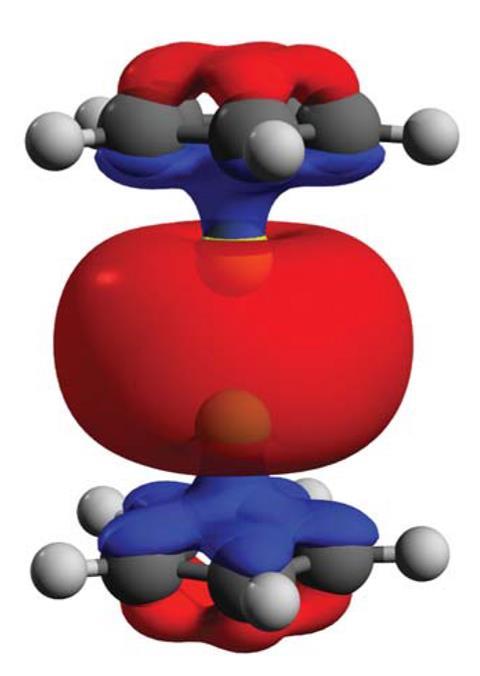
They found that diberyllocene featured two half-sandwich (cyclopentadienyl)beryllium units linked through a beryllium–beryllium bond. The Be–Be bond itself was 2.05Å in length – in line with previous quantum chemical predictions.
The researchers also concluded that diberyllocene was a reductant that could be used to synthesise other beryllium–metal bonds.
References
JT Boronski et al, Science, 2023, DOI: 10.1126/science.adh4419













No comments yet