Scientists in the US have used visible light to turn organic radicals into iron carbenes – attractive reactive intermediates with important applications in synthetic chemistry.1 The new approach, developed by a team working with Nobel laureate David MacMillan, overcomes safety concerns about carbene synthesis, making these compounds easier to handle in the lab.
Metal carbenes are of interest to chemists because they can perform chemistry that is nearly impossible to achieve in other ways, says Nathan Dow from Princeton University in the US who participated in the study. ‘To many of us, cyclopropanation and bond-insertion reactions are practically synonymous with carbene chemistry – and the molecules that are made with these tools see use everywhere in science, especially in medicine.’
But organic chemists usually make carbenes from unstable or dangerous materials such as diazo reagents. ‘These compounds have to be decorated with specific stabilising groups and can still explode in your hands if you’re not careful,’ warns Dow. ‘Instead, our method can plug in amino acids, alcohols and other chemicals that we have lying around our labs as an alternative.’
The approach uses photoredox chemistry and involves two single-electron steps: addition of an oxygen- or nitrogen-substituted radical to an iron centre and reduction of the metal by a photocatalyst. ‘This gives an iron compound which can form carbenes via alpha-elimination, akin to what happens when nitrogen is lost during diazo chemistry,’ explains Dow. But as the radicals come from simpler materials, the alpha-elimination reaction happens with unusual leaving groups to form carbenes under very mild conditions.
‘Depending on what we plug in, this method will turn some of nature’s building blocks, like lactic acid or alanine, into rare carbenes with alkyl groups – known as the ‘donor’ class – or it can make fluorine-based versions from everyday solvents like trifluoroethanol,’ says Dow. He adds that once formed, these intermediates can either create cyclopropanes and related drug scaffolds by reacting with alkenes, or undergo P–H, S–H or N–H bond-insertion reactions.
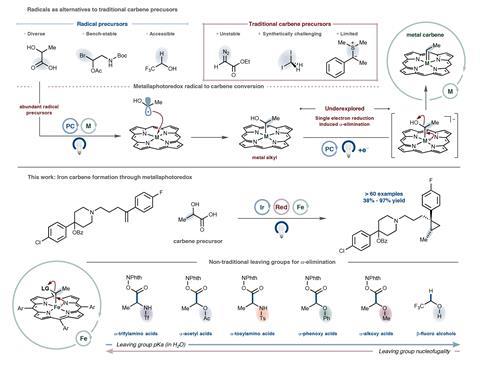
As carbene chemistry is widely used to make drugs, natural products and other essential compounds, Dow believes that the new strategy could help chemists to produce the molecules they need more easily and much more safely. ‘With photochemistry, we can also access unique types of carbenes and carbene precursors, and this could let us streamline how new therapeutic candidates or other next-generation molecules of interest are made.’
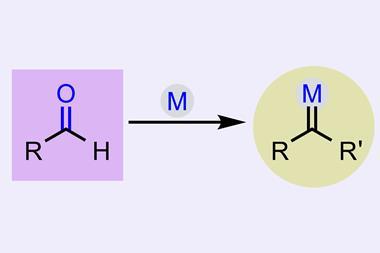
David Nagib from Ohio State University in the US, who recently developed a method that uses aldehydes instead of diazo compounds to make carbenes2, is happy to see renewed excitement in new classes of carbene reactivity. ‘The discovery of new mechanisms and approaches, such as by this photocatalytic strategy, will be key to more fully harnessing the unique and powerful reactivity of carbenes,’ he says.
Dow says chemists will welcome any method that provides access to safer and more reliable carbenes. He believes that a combination of different strategies could offer the best solution. ‘Rather than any one method bringing us over the mountain, I think of our one-electron approach as complementary to what’s already out there because it lets us consider an entirely distinct range of chemicals – amino acids, alcohols and so on – as viable carbene sources for the first time.’
References
1 BT Boyle et al, Nature, 2024 (DOI: 10.1038/s41586-024-07628-1)
2 L Zhang et al, Science, 2022, 377, 649 (DOI: 10.1126/science.abo6443)







No comments yet