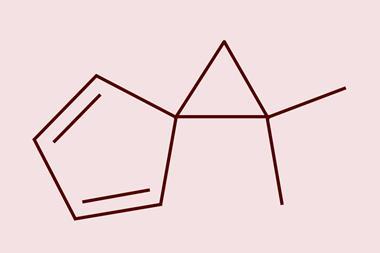
A new synthetic strategy uses a simple salt – iron chloride – to catalyse the creation of carbenes from commercial aldehydes. The carbenes quickly react with different alkenes to yield a selection of cyclopropanes ‘with a remarkable structural diversity’, according to Marc Montesinos Magraner, an expert in synthetic chemistry at the University of Valencia, Spain, who wasn’t involved in the study. Such cyclopropanes are ‘really difficult to prepare with existing methods’, but are common in medicines.
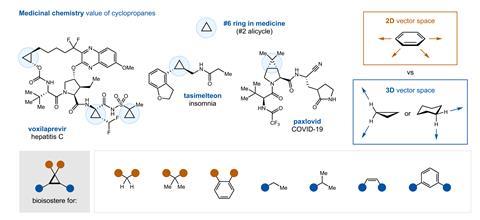
This could allow medicinal chemists to design and develop more diverse drug structures, explains lead author David Nagib from Ohio State University in the US. The new route to create carbenes provides ‘rapid access to many different classes of cyclopropanes – with varying substation patterns’, he explains. ‘We expanded on our strategy for harnessing carbenes, [and] add them to many different class of alkenes … to access smaller, more drug-like cyclopropanes.’
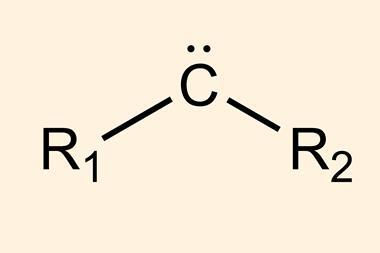
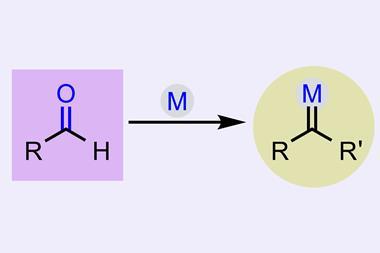
Carbenes constitute an unusual divalent form of carbon. ‘Carbenes are inherently unstable … [and] will do anything to increase their valency, because carbon wants to be tetravalent,’ explains Chris Uyeda, an expert in carbene reactivity at Purdue University, US. ‘Carbenes can’t be isolated or bottled … they must be generated on demand from a stable precursor,’ he explains. Nagib and co-workers selected simple and easily accessible aldehydes to generate carbenes, a solution that’s safer and easier than state-of-the-art alternatives. ‘Aldehydes are one of the most important feedstocks in organic chemistry,’ says Montesinos. ‘This methodology expands the synthetic potential of such a functional group,’ he says. ‘It’s a much more stable alternative and, at the same time, greatly expands the scope in terms of the structural diversity of carbenes and cyclopropanes.’
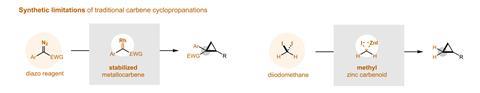
Otherwise, ‘the most common approach to create carbenes is the decomposition of diazocompounds, which are toxic and potentially explosive’, explains Montesinos. This leads to ‘enormous technical challenges associated with the scales needed to manufacture pharmaceuticals’, according to Uyeda. Sometimes, synthetic chemists solve this by adding stabilising electron-withdrawing groups to diazocompounds, but such a solution limits the structural diversity of the cyclopropanes. Other alternatives ‘are low yielding or require strong bases, incompatible with common functional groups’, adds Uyeda. In this case, ‘the major advance is the ability to use non-stabilised carbenes … [to] make cyclopropanes that were difficult to access in the past’.
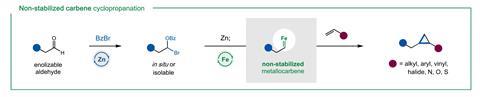
Another attractive aspect of this alternative method to make carbenes is the simplicity of the catalyst – iron(II) chloride. ‘We’re excited that such simple metal salts – without a ligand even – are suitable catalysts for [this] reaction,’ says Nagib. ‘The use of earth-abundant metal catalysts … suggests the synthetic strategy [could] be easily scaled into industrial settings,’ he adds. According to Uyeda, who has also developed cyclopropanation methods based on abundant metals, such as cobalt and nickel, iron catalysts have been largely overlooked. But ‘it’s an ideal metal, because it’s both inexpensive and non-toxic’, he explains. Moreover, the lack of ligands lowers the cost of the overall process, which could encourage scale-up and commercialisation.
References
BM DeMuynck et al, Chem, 2024, 10, 1-13, DOI: 10.1016/j.chempr.2024.01.006











No comments yet