Making macrocycles with twisted topologies
Researchers from Korea and Japan have put a new twist on aromaticity, synthesising the largest Möbius aromatic molecule to date.
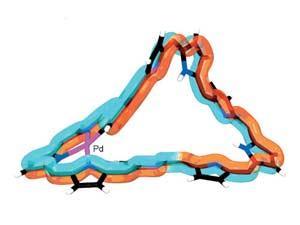
Like Möbius strips, Möbius aromatic molecules have a half-twist in their structure. The phenomenon was theoretically predicted in 1964 for rings large enough to undergo such a twist – around 20 atoms. However, such large, flexible rings are difficult to synthesise, and are likely to untwist, so the first stable Möbius aromatic molecule wasn’t made until 2003.
Now researchers have created the largest Möbius aromatic porphyrin known. By complexing the molecule with a palladium atom, the team reduced the ring’s flexibility during its synthesis, creating a macrocycle containing 46 carbon atoms – 12 more than the previously largest Möbius aromatic molecule.
The group is now exploring the synthesis of even larger porphyrins to study their chemical behaviour, and their optical and redox properties.
![Top view of the single-crystal X-ray diffraction structure of a dithienothiophene (DTT)-bridged [34]octaphyrin](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/3/6/2/132362_Bicyclic-Baird-type-aromaticity-fig-1-c.jpg)








No comments yet