Molecule undergoes unprecedented topology switch from Möbius aromatic to Hückel anti-aromatic when immersed in polar solvent
Scientists in Japan have developed a system that can switch its topology from being Möbius to Hückel and back again. Not only was this type of reversible switch unexpected, it could also be triggered by external stimuli such as a polar solvent.
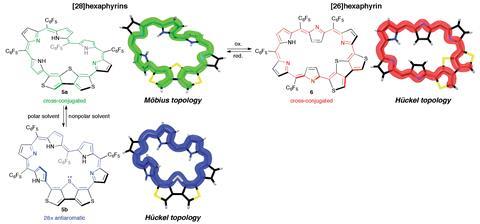
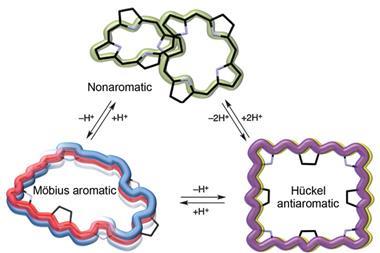
A cyclic molecule is Möbius aromatic if it has [4n]π electrons and anti-aromatic (destabilised) if it has [4n+2]π electrons. The opposite is true of Hückel systems – a system with [4n+2] conjugated electrons is aromatic and a [4n]π electron system is anti-aromatic. Aromaticity brings stability, so you would expect [28]hexaphyrins – having [4n]π electrons – to adopt an aromatic Möbius topology.
However, this is not what happened when a team from Japan immersed [28]hexaphyrin in dimethylfomamide (DMF). The NH protons of [28]hexaphyrin formed hydrogen bonds with the oxygen atoms of the polar solvent, which induced a conformational change to the anti-aromatic Hückel topology. The hydrogen bonds helped stabilise the supposedly unstable anti-aromatic system.
Key to achieving this reversible switching was incorporating a single dithienothiophene unit into the structure. Previous researchers had incorporated two such units to obtain different π-circuits depending on the oxidation state of sulfur in the dithienothiophene unit; however, as changing an oxidation state requires a chemical reaction, reversible π-switching was impossible.
They also showed they could use a redox reaction to convert [28]hexaphyrin into [26]hexaphyrin, with a different Hückel topology. This was neither aromatic nor anti-aromatic, but nonaromatic – the next challenge for the scientists is to determine why it prefers this conformation over an aromatic one.
![Top view of the single-crystal X-ray diffraction structure of a dithienothiophene (DTT)-bridged [34]octaphyrin](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/3/6/2/132362_Bicyclic-Baird-type-aromaticity-fig-1-c.jpg)









No comments yet