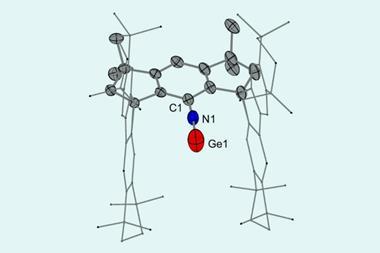
Researchers in India have prepared an all-boron analogue of benzene. It consists of a planar six-membered boron ring that’s stabilised by a single osmium pentamethylcyclopentadienyl complex.
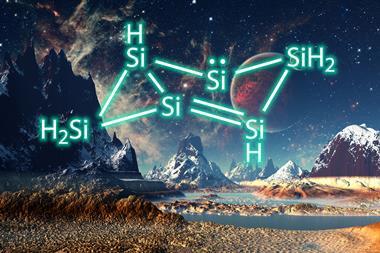
Boron’s electron deficiency makes it prefer three-dimensional polyhedral structures over chains and rings. In 2019, US researchers reported the first boron analogue of a metallabenzene – a six-membered ring with five boron atoms and one rhenium atom, plus an extra boron atom in the middle. Over the past 30 years, scientists have made planar rings containing three, four and five boron atoms by stabilising them with main group or transition metal fragments. While attempts at six-membered boron rings have produced structures that are either not truly planar or require heavy stabilisation with multiple metals or bulky ligands.
Now, Sundargopal Ghosh from the Indian Institute of Technology Madras and Eluvathingal Jemmis from the Indian Institute of Science Bangalore and their colleagues have synthesised the metallocene [Cp*Os(η6-B6H11)] by pyrolysing [BH3·SMe2] with [Cp*OsBr2]2.
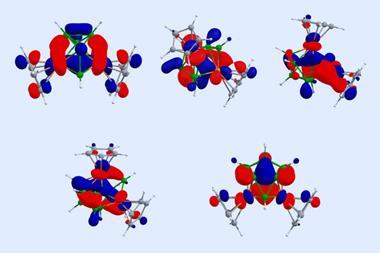
Computational studies and bonding analysis show that the metallocene’s B₆H₁₁ ring is isoelectronic with the benzenyl cation radical [C6H6]+ and aromatic in nature, making it comparable to benzene in sandwich complexes.
The team also identified two smaller all-boron rings, [Cp*Os(η⁵-B₅H₁₂)], and [Cp*Os(η⁴-B₄H₉)], as reaction intermediates.
The researchers say their findings open up fresh possibilities in organometallic chemistry, paving the way for new catalysts and electronic materials.
Correction: This article was updated on 25 November 2025 to clarify that B₆H₁₁ not B₆H₁₁- is isoelectronic to [C6H6]+







![An image showing a [M2(BzN6-Mes)]n− complex](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/4/0/8/514408_index_128835.jpg)








No comments yet